Geoffrey W. Zehnder and Changbin Yao
Department of Entomology
Auburn University, AL 36849
John F. Murphy, Edward J. Sikora and Joseph W. Kloepper
Department of Plant Pathology
Auburn University, AL 36849
David J. Schuster and Jane E. Polston
University of Florida, IFAS
Gulf Coast Research and Education Center
Bradenton, FL 34203
Plant disease caused by insect-transmitted pathogens are among the most serious production problems encountered by vegetable growers. Effective insecticidal control of insect-borne disease is problematic because most plant disease vectors are highly mobile insects and may colonize fields rapidly before growers are aware of their presence. In addition, even low numbers of insects may result in high field incidence of disease, as occurs with cucumber beetles and bacterial wilt disease (Yao et al. 1996). In Alabama, insecticides were completely ineffective to prevent a serious epidemic of cucumber mosaic virus (CMV) on tomato (Sikora et al. 1998). CMV is transmitted by aphids in a nonpersistent manner, meaning that an aphid vector can acquire and transmit virus in seconds. Thus insecticides do not act quickly enough to prevent inoculation. Our research during the past six years has been directed towards microbe-induced resistance as an alternative strategy for management of insect-transmitted diseases.
Plants have evolved complex and varied defense mechanisms for protection against herbivory and disease. These mechanisms may be constitutive (e.g., active throughout the plant’s life) or induced following attack by herbivores or pathogens. Recent studies have suggested that inducible defenses in plants may have selective advantages over constitutive defenses (Agrawal 1998 and references therein). While inducible defenses are often localized at the site of attack, plant defense mechanisms may be activated systemically throughout the plant following a localized infection or attack (Kessman et al. 1994). One of the first published reports of systemic resistance in plants was by Chester (1933), who used the term "acquired physiological immunity". Later, Ross (1961) reported that tobacco plants exhibited "systemic acquired resistance" following local infection with tobacco mosaic virus. Other terms that have been used to describe systemic resistance in plants include "translocated resistance" (Hubert and Helton 1967), "plant immunization" (Ku_ 1987), and "induced systemic resistance" (Hammerschmidt et al. 1982). In this report, we will use the latter term, or ISR, which can be defined as the process of active (systemic) protection of a plant dependent on the host plant’s physical or chemical barriers activated by an inducing agent when applied to a single part of the plant (Kloepper et al. 1992). Various "classical" inducers of ISR have been reported, including pathogens, attenuated pathogens, synthetic chemicals, and metabolic products of hosts or infectious agents (Liu et al. 1995, and references therein); however reports of induced resistance in the field by classical inducing agents have been infrequent.
Kloepper and Schroth (1978) reported that certain root-colonizing bacteria could promote radish growth in greenhouse and field trials and named the bacteria plant growth-promoting rhizobacteria (PGPR). Results of early studies with PGPR also demonstrated control of soilborne pathogens (Défago et al. 1990, Grusiddaiah et al. 1986, Ordentlich et al. 1987). PGPR act as antagonists against soil pathogens through competition (Elad and Chet 1987), or production of bacterial metabolites deleterious to the pathogen (e.g., siderophores, HCN, antibiotics) (Kloepper and Schroth 1978, Thomashow and Weller 1988, Weller 1988). More recently, three laboratories independently demonstrated that certain PGPR strains protected plants through mechanisms associated with ISR against pathogens that cause foliar disease symptoms (Alstrom 1991, Van Peer et al. 1991, Wei et al. 1991). Subsequent research at Auburn University has demonstrated PGPR-ISR against various fungal and bacterial pathogens of cucumber (Liu et al. 1995a,b). Following these studies, we were interested in testing some of the same PGPR strains for ISR against three intractable insect-transmitted diseases; bacterial wilt of cucurbits caused by Erwinia tracheiphila, and cucumber mosaic virus and tomato mottle geminivirus on tomato.
Experiments with PGPR-ISR against Cucumber Beetles and Bacterial Wilt of Cucurbits
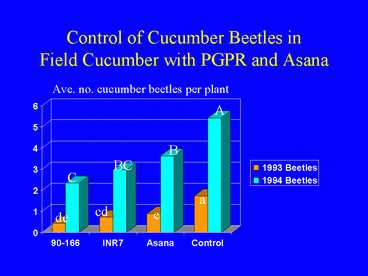
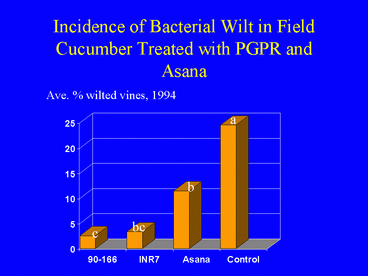
Field experiments in cucumber demonstrated that plants grown from seed treated with PGPR sustained significantly lower populations of cucumber beetles, Diabrotica undecimpunctata howardi and Acalymma vittatum, and lower incidence of bacterial wilt disease (Figs. 1, 2 and 3) compared with nontreated control plants and plants sprayed weekly with the insecticide esfenvalerate (Zehnder et al. 1997a). Free choice greenhouse experiments with cucumber beetles were conducted in which beetles that had acquired the wilt pathogen were released in screen cages (Fig. 4) and allowed to feed on PGPR-treated or nontreated plants. Cucumber beetle feeding damage on cotyledons and stems and incidence of wilt symptoms were significantly lower on PGPR-treated plants than on nontreated plants (Zehnder et al. 1997b, Figs. 5 and 6).




Role of Cucurbitacin in Induced Resistance in Cucumber
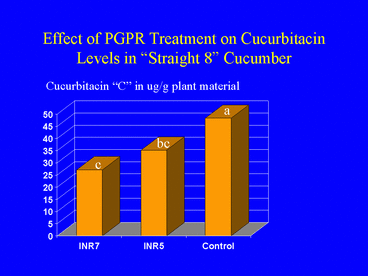
Because cucumber beetle feeding behavior is strongly influenced by cucurbitacins, a group of triterpenoid plant metabolites that occur in the plant family Cucurbitaceae (Chambliss and Jones 1966), and previous studies demonstrated a positive correlation between cucurbitacin content and cucumber beetle feeding (Ferguson et al., 1983), we hypothesized that plants induced by PGPR treatment contain a reduced concentration of cucurbitacins. To test this, we subjected cotyledon leaves of PGPR-treated and nontreated cucumber plants to HPLC analysis for cucurbitacin. We tested two cucumber cultivars; one with high and one with low levels of cucurbitacin. Results indicated that PGPR-treated plants of both cultivars contained significantly lower levels of cucurbitacin than nontreated plants (Zehnder et al. 1997b; Fig. 7). This suggested that a mechanism for PGPR-induced resistance against cucumber beetle feeding may involve a change in the metabolic pathway for cucurbitacin synthesis. Support for this hypothesis comes from previous studies demonstrating that metabolic pathways for cucurbitacin and other plant defense compounds share similar precursors (Balliano et al. 1982).
Experiments with PGPR-ISR against CMV on Tomato
Our success with PGPR-ISR in cucumber led us to expand our research to address cucumber mosaic virus (CMV) infection of tomato. A severe epidemic of CMV in fresh market tomato in North Alabama resulted in an estimated 25% yield loss in that region (Sikora et al. 1998). CMV is a particularly difficult virus to manage, due in part to its extensive host range and ability to be transmitted by more than 65 aphid species. Moreover, there is a lack of genetically resistant fresh-market tomato varieties to CMV infection, so management options are limited. Thus, tomato growers are in need of alternative management strategies, particularly those that are environmentally sound and easily implemented.

A series of greenhouse experiments was conducted at Auburn to evaluate 26 PGPR strains for ISR against CMV in tomato. These strains were selected based on their ability to induce resistance against other pathogens of cucumber and tomato. In these experiments, PGPR cultures were centrifuged and the tomato seeds mixed with the bacterial pellet before planting into plastic pots with planting mix. After two weeks of growth, tomato plants were transplanted into new plastic pots containing planting mix, and PGPR suspension treatments (100 ml containing approximately 5 x 108 cfu/ml) were poured into each pot immediately after transplanting. CMV inoculum was prepared from fresh tobacco leaves infected with CMV. The CMV extract was rub-inoculated onto the first two tomato leaves/plant one week after transplanting. Plants were examined daily for CMV symptoms (Fig. 8); these typically include leaf distortion, chlorosis and mosaic symptoms on leaves, and a general stunting of the plant. The number of symptomatic leaves per plant and the number of plants with severe symptoms (e.g., >2/3 of symptomatic leaves) were recorded. After the initial screening experiment, 14 of the best PGPR strains were selected for further evaluation. The range in the percentage of plants exhibiting CMV symptoms 10 days after inoculation in the 14 PGPR treatments was 40-70%, compared to 100% of plants with symptoms in the treatment where plants were inoculated with CMV but not treated with PGPR. In a second series of greenhouse experiments to evaluate the 14 PGPR strains, treatment with 8 PGPR strains resulted in 30-40% plants with symptoms, compared to 90% of nontreated plants with symptoms. Finally, in a third series of greenhouse experiments, 4 of the 8 PGPR strains were chosen for further evaluation in field experiments. The percentage of plants with CMV symptoms in the 4 PGPR treatments ranged from 14.7 to 22.1%, compared with 75.8% of plants with symptoms in the plants without PGPR treatment.
Field experiments were done in 1996 and 1997 to evaluate the 4 PGPR strains, a disease control (mechanical inoculation with CMV; no PGPR) and a healthy control (no CMV inoculation; no PGPR). There were 6 replications per treatment arranged in a randomized block design, each consisting of 15 tomato plants (single row plots). For PGPR treatments in the field experiments, `Mountain Pride’ tomato seeds were mixed with the PGPR pellet (as described above). In addition to seed treatment, a PGPR soil drench was poured around the base of each plant immediately after transplanting (when plants were in the two leaf stage). All plants except those in the healthy control treatment were inoculated with CMV, as described above, one week before transplanting in the field. All plants in each treatment were examined weekly for virus symptoms using the following rating scale: 0, no symptoms; 2, leaf puckering or curling just beginning; 4, 50% of leaves on plant appear puckered or curled; 6, mosaic symptoms just beginning; 8, 50% of leaves showing mosaic symptoms; 10, 100% of leaves showing mosaic symptoms. Disease severity values were calculated using the formula:
Disease severity (Y) = [S(rating no.) (no. plants in rating category)(100)]
(Total no. plants)(highest rating value)
Disease progression over time was measured using a formula for calculating the area under the disease progress curve (AUDPC):
AUDPC = S [(0.5)(Yi + 1 + Yi)(Ti + 1 + Ti)]
where Y = disease severity at time T, and I = the time of the assessment (in days numbered sequentially beginning with the initial assessment).
In addition to virus symptom rating, leaves from the upper plant canopy were collected from each plant 32 days after transplanting in the field (90 leaf samples per treatment). Leaf samples were subjected to indirect enzyme-linked immunosorbent assay (ELISA). Height of plants was measured 30 days after transplanting in the field. Marketable (non-damaged and mature) tomato fruit were weighed on 6 harvest dates during the season.
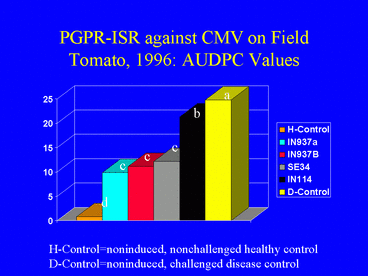
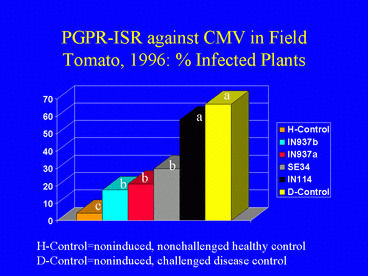
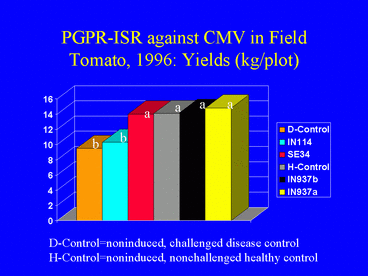
In the 1996 field experiment, AUDPC values, indicating disease symptom progression over time, were significantly lower in all PGPR treatments compared with the disease control (Fig. 9). Similarly, ELISA values in all PGPR treatments, and the percentage of infected plants (Fig. 10) (based on ELISA) in 3 PGPR treatments, were significantly lower than in the disease control. The percentage of infected plants in the disease control treatment was over 3-fold greater than in the IN937a and IN937b treatments. Plant height measurements taken 30 days after transplanting indicated that plant growth in the PGPR treatments was greater than in the disease control. The increased growth may have resulted from PGPR-induced resistance against CMV, PGPR-induced growth promotion, or both factors combined. Importantly, yields in the SE34, IN937a and IN937b treatments were significantly greater than in the disease control (Fig. 11).
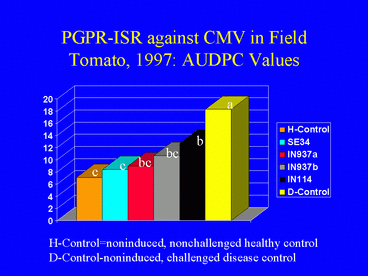
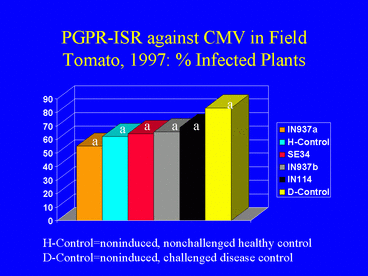
As in 1996, results of the 1997 field experiment indicated that AUDPC values (Fig. 12) and ELISA absorbance values were significantly lower in the PGPR treatments than in the disease control. Overall, the percentage of plants infected with CMV was higher in 1997 than in 1996 (Fig. 13). In 1997, 62.2% of the nonchallenged, healthy control plants were infected, suggesting that naturally-occurring aphids acquired the virus by feeding on the mechanically-inoculated plants, and subsequently inoculated other plants, including those in the healthy control treatment. This could have resulted in the higher incidence of CMV in 1997. Although the percentages of infected plants in the PGPR treatments were lower than in the disease control, differences were not significant. Plant growth was significantly greater in the PGPR treatments compared with the disease control, but average tomato yields were not significantly different among treatments.
Field Experiment with PGPR-ISR against Tomato Mottle Geminivirus on Tomato
Tomato mottle, caused by the tomato mottle virus (ToMoV), poses a major threat for both transplant and field production of tomato in west-central and south-west Florida (Abouzid et al. 1992, Polston et al. 1993). ToMoV is in the geminiviridae family and is transmitted by adult sweet potato whiteflies, Bemisia tabaci, biotype B (also known as the silverleaf whitefly, Bemisia argentifolii). Symptoms of ToMoV in field grown tomatoes include chlorotic mottling and upward curling of leaflets, and an overall reduction in plant height and in the number and size of fruit (Polston et al. 1993). Similar to the CMV pathosystem, management of ToMoV is very difficult. Tomato varieties resistant to ToMoV are not yet commercially available, and insecticides have not provided effective management; in part because of the development of insecticide-resistant whitefly biotypes. Prompted by our findings that PGPR-ISR successfully protected tomato from infection by CMV, trials were planned to evaluate PGPR for ISR in tomato against ToMoV.
Field experiments were conducted during the fall tomato growing season at the University of Florida Gulf Coast Research and Extension Center in Bradenton, Florida. Tomatoes grown at the Center were exposed to high levels of natural whitefly infestation and ToMoV infection. Spores of PGPR strains IN937b and SE34 (two of the strains used in experiments with CMV described above) were produced in culture and formulated as both a seed treatment and a powder by Gustafson Corp., Plano, TX. The PGPR powder was diluted with water according to manufacturer recommendations and incorporated into the planting mix before seeding. Seed treatment and powder formulations of each PGPR strain were evaluated in single row plots; treatments were replicated four times. At 40 days after planting, each plant in each treatment plot was rated for disease severity using a scale of 0 to 2.5, and all samples were analyzed for ToMoV DNA by dot blot analysis (Polston et al. 1993). Leaf samples for analysis of ToMoV DNA were also collected 80 days after planting. The disease rating scale was as follows: 0 = no symptoms; 0.5 = early stages of chlorosis of young leaf bases; 1.0 = obvious chlorosis/mottle of leaves on any of the tomato plant stems; 1.5 = obvious chlorosis/mottle of leaves over most of the plant; 2.0 = serious chlorosis/mottle of leaves and deformation and leathery appearance of leaves; 2.5 = severe chlorosis/mottle of leaves and deformation and leathery appearance of leaves and plants severely stunted. Tomatoes were harvested from all plots 80, 94 and 108 days after transplanting.
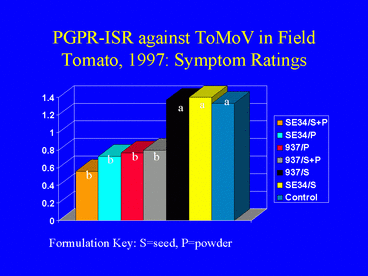
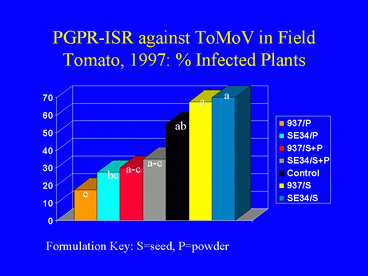
The IN937b and SE34 PGPR strains both provided protection against ToMoV in the Florida tomato field experiment. Visual symptom ratings and dot blot analysis of leaves at 40 days after transplanting indicated that the PGPR powder and powder + seed formulations were more effective for ISR against ToMoV than the PGPR seed formulations (Fig. 14). The severity of virus symptoms was significantly lower in all PGPR powder and powder + seed treatments than in the nontreated control, but symptoms were not significantly different between the seed-only treatments and the control (contrast analysis; a = 0.05). There were no significant differences in symptom ratings between the PGPR powder only and the PGPR seed + powder formulations. Contrast analysis of the percentage of plants testing positive for ToMoV DNA from leaf samples collected 40 days after planting generated similar results (Fig. 15). By 80 days after planting, most plants in all treatments were infected with ToMoV, and differences in the percentages of infected plants among treatments were not significant.
At the first harvest date at Bradenton (80 days after transplanting), tomato yields were higher in PGPR powder or seed + powder treatments than in the control or seed-only treatments; however yield differences were statistically significant only between the 937b powder treatment and the control. Analysis of tomato yield data from harvests at 94 and 108 days after transplanting did not indicate a significant effect of PGPR treatment on tomato yield. We suspect that PGPR-ISR against ToMoV occurred in the early stages of infection, but that continual whitefly infestation of plants and inoculation of the virus eventually overcame the induced resistance response.
Discussion
The results with CMV provide a preliminary indication that PGPR-induced ISR against CMV on tomato, previously reported from greenhouse experiments (Raupach et al. 1996), can be obtained under field conditions. It is known that virus symptoms and effects on yields are most severe when plants are infected with virus in early growth stages. Therefore, we were encouraged by the 1996 field results showing that virus symptoms were reduced and tomato yields were not affected on PGPR-treated plants that were mechanically challenged with virus even before transplanting in the field. In 1997, when natural CMV infection by aphids apparently supplemented infection by mechanical infection, PGPR treatment reduced the severity of virus symptoms, but the effects of PGPR treatment on the percentage of infected plants and on tomato yields was not as great as in 1996. This may indicate that the impact of PGPR-ISR may be most apparent when the levels of virus inoculum are low or moderate, and that PGPR-induced plant defense mechanisms are not as effective in the presence of high viral inoculum. This hypothesis is corroborated by the fact that we have not observed PGPR-ISR against naturally-transmitted CMV in trials conducted on a north Alabama tomato farm where the incidence of CMV infection was extremely high.
The results with ToMoV in Florida following the CMV experiments in Alabama demonstrate that specific PGPR strains can provide broad spectrum protection in field tomato against viruses in different groups with different insect vectors; i.e., CMV with aphids and ToMoV with whiteflies. The observed level of PGPR-ISR against ToMoV was encouraging given that the PGPR strains used in the Florida trial were selected based on screening for activity against CMV and not ToMoV. This illustrates the potential of PGPR to provide broad spectrum protection against several pathogens. The levels of whitefly infestation and ToMoV inoculum at the Bradenton field experiment site were greater than what typically occurs in commercial tomato fields. Thus it is possible that the level of PGPR-induced protection against ToMoV in commercial tomato fields may be greater than might be expected based on our experimental results. In addition, vegetative cell treatments of PGPR were used in our previous experiments in tomato. The PGPR spore seed and powder formulations used in the Florida trial had not previously been tested.
Results of the Florida experiment also indicate that the PGPR powder formulations were more effective for ISR against ToMoV than the treatments where PGPR was applied only to the seed. Although we have not compared the speed of PGPR germination and root colonization in the two formulations, it is possible that these events occurred earlier in the powder formulation treatments than in the seed treatments resulting in an earlier ISR response. Formulations of PGPR powder provide a practical delivery system for ISR in crops because powder can be easily added to planting mix, or mixed with water and applied as a transplant drench application.
Our results with PGPR-ISR over the past six years have shown that PGPR can be an effective tool for crop protection against a variety of plant pathogens and, in specific cases (i.e., cucumber beetles and bacterial wilt), can protect plants from insect feeding and transmission of disease. Cooperative work with Gustafson, Inc., is underway to evaluate commercially-prepared PGPR seed treatments for cucumber, and it is possible that a PGPR seed treatment for cucurbit crops will be commercially available within the next few years. We will continue to evaluate the stability and durability of PGPR protection against plant viruses under diverse field conditions where natural infection by insect vectors occurs. Increased efforts to screen PGPR strains for ISR against other viruses, like ToMoV, will likely result in the identification of PGPR strains, or a combination of strains, that may be used effectively to reduce the impact of virus infection on plant health and yields.
Because specific PGPR strains have demonstrated ISR against multiple pathogens, it is possible that several PGPR strains could be identified for each vegetable crop to provide some level of protection against multiple pathogens of economic importance. We do not view PGPR as a universal solution for all crop protection needs. Rather, we believe that PGPR represent a potentially useful tool for environmentally sound pest management programs and a means to reduce our reliance on pesticides by exploiting the plant’s existing defense mechanisms.
References
- Abouzid, A.M., Polston, J.E., Hiebert, E. 1992. The nucleotide sequence of tomato mottle virus; a new geminivirus isolated from tomato in Florida. J. Gen. Virology 73: 3225-3229.
- Agrawal, A.A. 1998. Induced responses to herbivory and increased plant performance. Science 279: 1201-1202
- Alström, S. 1991. Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizoshpere pseudomonads. J. Gen. Appl. Microbiol. 37: 495-501
- Balliano, G., Caputo, O., Viola, F., Delprino, L., Cattel, L. 1982. The transformation of 10a-cucurbita-5,24-dien-3ß-ol into cucurbitacin C by seedlings of Cucumus sativus. Biochemistry 22: 909-913
- Défago, G., Berling, C.H., Burger, V., Haas, D., Kahr, G., Keel, C., Voisard, C., Wirthner, P.H., Wuthrich, B. 1990. Suppression of black root rot of tobacco by a Pseudomonas strain: Potential applications and mechanisms. Pp. 93-108, In D. Hornby, R.J. Cook and Y. Henis, eds. Biological Control of Soilborne Plant Pathogens, CAB International, Wallingford, England
- Chambliss, O., Jones, C.M. 1966. Cucurbitacins: specific insect attractants in Cucurbitaceae. Science 153: 1392-1393
- Chester, K. 1933. The problem of acquired physiological immunity in plants. Quart. Rev. Biol. 8: 129-151
- Ferguson, J.E., Metcalf, E.R., Metcalf, R.L., Rhodes, A.M. 1983. Influence of cucurbitacin content in cotyledons of cucurbitaceae cultivars upon feeding behavior of Diabroticina beetles. J. Econ. Entomol. 76: 47-51
- Gurusiddaia, S., Weller, D.M., Sarkar, A., Cooks, J.R. 1986. Characterization of an antibiotic produced by a strain of Pseudomonas fluorescens inhibitory to Gaeumannomyces graminis var. tritici and Pythium spp. Antimicrob. Agents Chemother. 29: 488-495
- Kessman, H., Staub, T., Hofmann, C., Maetzke, T., Herzog, G., Ward, E., Uknes, S., Ryals, J. 1994. Induction of systemic acquired disease resistance in plants by chemicals. Annu. Rev. Phytopathol. 439-460
- Kloepper, J.W., Schroth, M.N. 1978. Plant grotwh-promoting rhizobacteria in radish. pp 879-882. Proc. 4th Int’l. Conf. Plant Pathogenic Bact. Gilbert-Clarey, Tours, France
- Kloepper, J.W., Tuzun, S., and Ku_, J. 1992. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 2: 349-351
- Liu, L., Kloepper, J.W., Tuzun, S. 1995. Induction of resistance in cucumber by plant growth-promoting rhizobacteria: duration of protection and effect of host resistance on protection and root colonization. Phytopathology 85: 1064-1068
- Ordentlich, A., Elad, Y., Chet, I. 1987. Rhizosphere colonization by Serratia marcescens for the control of Sclerotium rolfsii. Soil Biol. Biochem. 19: 747-751
- Polston, J.E., Hiebert, E., McGovern, R.J., Stansly, P.A., Schuster, D.J. 1993. Host range of tomato mottle virus; a new geminivirus infecting tomato in Florida. Plant Disease 77: 1181-1184.
- Raupach, G.S., Liu, L., Murphy, J.F., Tuzun, S., Kloepper, J.W. 1996. Induced systemic resistance in cucumber and tomato against cucumber mosaic cucumovirus usnig plant growth-promoting rhizobacteria (PGPR).
- Sikora, E.J., Gudauskas, R.T., Murphy, J.F., Porch, D.W., Andrianifahanana, M., Zehnder, G.W., Bauske, E.M., Kemble, J.M., Lester, D.F. 1998. A multivirus epidemic of tomatoes in Alabama. Plant Disease 82: 117-120
- Tomashow, L.S., Weller, D.M. 1988. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J. Bacteriol. 170: 3499-3508
- Van Peer, R., Niemann, G.J. and Schippers, B. 1991. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 81: 728-733
- Wei, G., Kloepper, J.W., Tuzun, S. 1991. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 81: 1508-1512
- Weller, D.M. 1988. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 26: 379-407
- Yao, C., Zehnder, G., Bauske, E., Kloepper, J. 1996. Relationship between cucumber beetle (Coleoptera: Chrysomelidae) density and incidence of bacterial wilt of cucurbits. J. Econ. Entomol. 89: 510-514
- Zehnder, G., Kloepper, J., Yao, C., Wei, G. 1997a. Induction of systemic resistance in cucumber against cucumber beetles by plant growth-promoting rhizobacteria. J. Econ. Entomol. 90: 391-396.
- Zehnder, G., Kloepper, J., Tuzun, S., Yao, C., Wei, G., Chambliss, O., and Shelby, R. 1997b. Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomol. Exp. et Applicata 83: 81-85.