Gary D. Thompson, Scott H. Hutchins and Thomas C. Sparks
Dow AgroSciences LLC
9330 Zionsville Rd., Indianapolis, IN, 46268, USA
Introduction
The discovery and characterization of the soil actinomycete Saccharopolyspora spinosa represented a novel opportunity to develop a portfolio of progressive insect management tools (Thompson et al.1997, Sparks et al. 1998). Indeed, the discovery and subsequent development of this unique organism (Figure 1) has provided the world with an entirely new class of products - with Tracer* Naturalyte* Insect Control being the first to be commercialized.
The name is based on the utility of naturally produced metabolites that by definition possess rapid efficacy competitive with the best synthetic standards and safety profiles similar to benign biologicals. Spinosad is a mixture of the two most active naturally occurring metabolites (spinosyns A and D) produced by S. spinosa (Figure 2, Kirst et al., 1992).
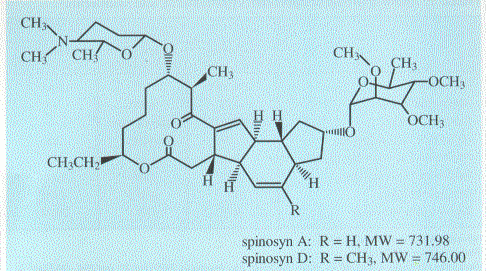
Structurally, these compounds are macrolides and contain a unique tetracyclic ring system to which two different sugars are attached. A unique mode of action coupled with a high degree of activity on targeted pests and low toxicity to non-target organisms (including many beneficial arthropods) make spinosad an excellent new tool for management of insect pests. Dow AgroSciences is aggressively pursuing additional spinosyns and other metabolites from natural organisms as future entries to the Naturalyte class. This paper provides an overview of the physical and biological properties of spinosad. When these properties are considered, in total, it is clear that Naturalyte does not conform to the previous characteristics of either chemical or biological insecticides.
Physical Properties and Environmental Fate
Spinosad is a secondary metabolite from the aerobic fermentation of S. spinosa on nutrient media. Following fermentation, spinosad is extracted and processed to form a highly concentrated conventional aqueous suspension for ease of use and distribution. Spinosad is a light gray to white crystalline solid with an earthy odor similar to slightly stale water. It has a pH of 7.74, is stable to metal and metal ions for 28 days, and has a shelf life of three years as formulated material. It is considered nonvolatile with vapor pressures around 10-10 mm Hg. Table 1 summarizes other physical and chemical properties of spinosyns A and D (Anonymous, 1996).
Table 1. Physical and chemical properties of spinosyns A and D.
| Spinosyn A | Spinosyn D | |
|---|---|---|
| Molecular Weight | 731.98 | 746.00 |
| Empirical Formula | C42H67NO16 | C41H65NO16 |
| Melting Point | 84 - 99.5°C | 161.5 - 170°C |
| Vapor Pressure | 2.4 x 10-10 | 1.6 x 10-10 |
| Solubility in Water at pH 5.0 | 290 ppm | 29 ppm |
| Solubility in Water at pH 7.0 | 235 ppm | 0.332 ppm |
| Solubility in Water at pH 9.0 | 16 ppm | 0.053 ppm |
| Octanol/Water
Partition Coefficient at pH 5.0 |
log P = 2.8 |
log P = 3.2 |
| Octanol/Water
Partition Coefficient at pH 7.0 |
log P = 4.0 |
log P = 4.5 |
| Octanol/Water
Partition Coefficient at pH 9.0 |
log P = 5.2 |
log P = 5.2 |
The degradation of spinosad in the environment occurs through a combination of routes, primarily photodegradation and microbial degradation to its natural components of carbon, hydrogen, oxygen and nitrogen. The half-life of spinosad degraded by soil photolysis is 9-10 days. It is less than 1 day for aqueous photolysis and leaf surface photolysis results in a half-life of 1.6 to 16 days. The half-life of spinosad degraded by aerobic soil metabolism in the absence of light is 9-17 days. Hydrolysis does not contribute significantly to degradation as spinosad is relatively stable in water at a pH of 5-7 and has a half-life of at least 200 days at a pH of 9. The leaching potential of spinosad is very low due to a moderate Kd (5-323), low to moderate water solubility and short residual in the environment. Thus, it does not pose a threat to groundwater when used properly and no buffer zones are required by the United States Environmental Protection Agency (Saunders and Bret, 1997).
Nontarget Toxicology
Table 2 summarizes acute mammalian, aquatic and avian toxicology (Anonymous, 1996). Spinosad is relatively low in toxicity to mammals and birds and is only slightly toxic to fish.
Table 2. Acute mammalian, aquatic and avian toxicity of spinosad.
| Species | Test | Result | EPA Category |
|---|---|---|---|
| Mammalian | |||
| Rat (male/female) | Acute oral LD50 | 3738/>5000 mg/kg | Caution (IV) |
| Mouse | Acute oral LD50 | >5000 mg/kg | Caution (IV) |
| Rabbit | Acute dermal LD50 | >5000 mg/kg | Caution (IV) |
| Rat | Acute inhalation LC50 | >5 mg/kg | Caution (IV) |
| Rabbit | Eye irritation | slight, clearing in 2 days | Caution (IV) |
| Rabbit | Skin irritation | no irritation | Caution (IV) |
| Guinea pig | Dermal sensitization | no sensitization | N. A. |
| Aquatic | |||
| Daphnia | 48 hr acute LC50 | 92.7 mg/L | Slightly toxic |
| Grass shrimp | 96 hr acute LC50 | >9.8 mg/L | Slightly toxic |
| Carp | 96 hr acute LC50 | 5.0 mg/L | Moderately toxic |
| Bluegill | 96 hr acute LC50 | 5.9 mg/L | Moderately toxic |
| Sheepshead minnow | 96 hr acute LC50 | 7.9 mg/L | Moderately toxic |
| Rainbow trout | 96 hr acute LC50 | 30.0 mg/L | Slightly toxic |
| Avian | |||
| Bobwhite quail | Acute oral LD50 | >2,000 mg/kg | Practically non-toxic |
| Mallard duck | Acute oral LD50 | >2,000 mg/kg | Practically non-toxic |
| Bobwhite quail | 5 day dietary LC50 | >5,000 mg/kg | Practically non-toxic |
| Mallard duck | 5 day dietary LC50 | >5,000 mg/kg | Practically non-toxic |
In addition, chronic toxicology tests in mammals have shown that spinosad is not carcinogenic, teratogenic, mutagenic or neurotoxic. Spinosad exhibits wide margins of safety to many beneficial insects and related organisms (Schoonover and Larson, 1995). Spinosad has relatively low activity against predaceous beetles, sucking insects, lacewings and mites. Table 3 demonstrates the reduced activity of spinosad on some.
Table 3. Toxicity of spinosad and cypermethrin to selected beneficial organisms.
| Beneficial species | Spinosad LC50 | Cypermethrin LC50 |
|---|---|---|
| Honeybee, Apis mellifera | 11.5 ppm | 1.2 ppm |
| Whitefly parasitoid, Encarsia formosa | 29.1 ppm | 1.9 ppm |
| Minute pirate bug, Orius insidiosus | 200 ppm | 0.2 ppm |
| Lady beetle, Hippodamia convergens | >200 ppm | 0.2 ppm |
| Lacewing, Chrysopa rufilabris | >200 ppm | <0.2 ppm |
| Predaceous mite, Phytoseiulus persimilis | >200 ppm | <0.2 ppm |
Against lepidoptera targets the activity values for spinosad and cypermethrin generally overlap. It is extremely exciting to have this level of activity coupled with large margins of selectivity, for predacious insects, which are an important component of IPM programs. The topical acute activity of spinosad against honeybees is less than 1 µg per bee which places spinosad in the highly toxic to bees category of the EPA. However, once residues have dried completely, toxicity of foraging bees is considered negligible (Mayer and Lunden, 1998). There are minimal safety precautions and preharvest and reentry intervals for this reduced risk product.
Physiological Properties and Resistance Management
Spinosad demonstrates rapid contact and ingestion activity in insects which is unusual for a biological product. The mode of action of spinosad is characterized by excitation of the insect nervous system, leading to involuntary muscle contractions, prostration with tremors, and paralysis. These effects are consistent with the activation of nicotinic acetylcholine receptors by a mechanism that is clearly novel and unique among known insect control products. Spinosad also has effects on GABA receptor function that may contribute further to its insect activity. This mode of action is unique. Imidacloprid and other nicotinic receptor-based insecticides act at a different site than spinosad. Avermectin, although a natural product and a macrocyclic lactone, also acts at a different site than spinosad. No other class of products affects the insect nervous system with the same mode of action and no cross resistance to spinosad has been demonstrated (Salgado et al., 1997; Salgado, 1998; Salgado et al. 1998).
The unique mode of action, lack of cross resistance, selectivity that leaves predacious insects, and moderate residual profile result in a low probability of resistance development. This is particularly true if Naturalyte insect control products are brought into rotations with current and other new insect control products. However, the adaptability of insects has been proven over and over. Therefore, Dow AgroSciences is promoting resistance management of spinosad through labeling, education and marketing efforts in support of good stewardship and IPM practices but not because of any underlying concerns that would affect spinosad more than any other active ingredient.
In the field, spinosad activity is characterized by cessation of feeding and paralysis of exposed insects within minutes. However, these insects may remain on the plant for up to two days. For this reason, growers and scouts should wait a minimum of two to three days to evaluate control.
Foliar applications of spinosad are not highly systemic in plants although some translaminar movement in leaf tissue has been demonstrated. The addition of a penetrating surfactant increases translaminar movement and activity on pests that mine leaves (Larson, 1997). No phytotoxicity has been demonstrated with this product.
Spectrum of Activity and Labeling Efforts
Spinosad has been tested extensively on a global basis since 1990 (Carson and Trumble, 1997; Fouche et al., 1998; Kerns, 1996; Linduska et al., 1998; McLeod, 1998; Palumbo, 1997; Riley, 1998; Schuster, 1997, Stansly and Connor, 1998; Walgenbach and Palmer, 1997; Webb, 1998). Table 4 lists several of the insect pests against which spinosad is being labeled.
Table 4. Examples of Insect pests controlled by spinosad on current or future labels.
| Common name | Scientific name | gm/ha |
|---|---|---|
| European corn borer | Ostrinia nubilalis | 25 -50 |
| Tomato fruitworm | Helicoverpa zea | 40 - 100 |
| Cabbage Looper | Trichoplusia ni | 50 - 100 |
| Diamondback moth | Plutella xylostella | 15 -50 |
| Southern armyworm | Spodoptera eridania | 50 - 100 |
| Leafminers | Liriomyza spp. | 70 - 150 |
| Melon thrips | Thrips palmi | 70 - 100 |
| Fall armyworm | Spodoptera frugiperda | 25 - 100 |
| Beet armyworm | Spodoptera exigua | 50 - 100 |
| Colorado potato beetle | Leptinotarsa decemlineata | 25 - 80 |
| Imported cabbageworm | Pieris rapae | 50 - 100 |
| Tomato pinworm | Keiferia lycopersicella | 50 - 100 |
| Grape Berry Moth | Lobesia lobina | 25 - 50 |
| Cotton Leafworm | Alabama argillacea | 25-50 |
| American bollworm | Heliothis armigera | 50 -100 |
| Tomato hornworm | Manduca quinquemaculata | 40 - 100 |
| Western flower thrips | Frankliniella occidentalis | 70 - 100 |
In general, spinosad provides effective control of pests in the insect orders Lepidoptera, Diptera, and Thysanoptera. It is also effective for some species Coleoptera and Orthoptera that consume large amounts of foliage. Spinosad is generally not effective for control of most sucking insects, and mites but some use patterns are being investigated.
Product Labeling
Spinosad is currently labeled in the U.S. on the brassica vegetable group (broccoli, Chinese broccoli, Brussels sprouts, cabbage, Chinese cabbage - bok choy and napa, cauliflower, cavalo, collards, kale, kohlrabi, mizuna, mustard greens, mustard spinach, Chinese mustard cabbage - gai choy, and rape greens), fruiting vegetable group (eggplant, ground cherry, pepino, pepper, tomatillo, and tomato), leafy vegetable group (including head and leaf lettuce, celery, arugula, chervil, edible chrysanthemum, corn salad, cress, dandelion, dock, endive, fennel, parsley, garden purslane, radicchio, rhubarb, spinach, and Swiss chard), apples, almonds and citrus. Crops for which additional U. S. labeling is being pursued include cucurbits, legumes, sweet corn, potatoes, and strawberries. Table 5 lists the global registration status at the time this paper was
Table 5. Current and Pending Naturalyte* Insect Control Registrations
| County | Trademarks | Crops | First Registration** |
|---|---|---|---|
| Argentina | Tracer*, Success* | Cotton, Tomato | 1998 |
| Australia | Tracer | Cotton | 1998 |
| Laser | Brassica | 1999 | |
| Success | Vegetables | 2000 | |
| Benelux | - | Pome fruit, Vegs. | 2002 |
| Bolivia | Tracer | Cotton/Soybeans | 1998 |
| Brazil | Tracer, Credence* | Tomato, Corn | 1998 |
| Soybean, Cotton, | 1999 | ||
| Canada | Success | Apples, Potatoes | 1999 |
| Stone Fruit | 2000 | ||
| Chile | Success | Nectarines, Tomato | 1998 |
| China | Success
Tracer |
Vegetable
Cotton |
1998
1998 |
| Columbia | Tracer 120SC | Flowers, Cotton, Begs. | 1998 |
| Cyprus | Tracer 48 SC | Vegetables, Potatoes | 1998 |
| Ecuador | Tracer | Corn, Vegetables | 1998 |
| France | - | Vines | 2001 |
| Ornamentals | 2001 | ||
| Germany | - | Flybait, Ornamentals | 2000 |
| Greece | - | Vines, Pome fruit, Vegs. | 2001 |
| Guatemala | Tracer | Cotton
Cucurbits, Brassica |
1998
2000 |
| Honduras | Tracer | Cotton | 1998 |
| Indonesia | Success | Vegetable | 1998 |
| Israel | _ | Vines, Vegetables | 1998 |
| Italy | _ | Ornamentals, Vegs. | 2001 |
| Grapes, Pome fruit | 2001 | ||
| Japan | Spinoace*, Naturalyte
Caribstar* |
Turf
Brassica Peach, Pear |
1999
1999 2001 |
| Korea | Boomerang | Cucumber | 1997 |
| Cabbage, Rose | 1998 | ||
| Lebanon | - | Vegetable, Potatoes | 1998 |
| Malaysia | Success 25SC | Vegetables | 1998 |
| Mexico | Tracer, Success | Cotton | 1997 |
| Fruiting Vegetables | 1998 | ||
| Brassica & Leafy Veg. | 1998 | ||
| New Zealand | Success | Vegetables | 1998 |
| Philippines | Success 25 SC | Vegetable | 1998 |
| Spain | - | Vines | 2001 |
| Pepper, Tomatoes | 2001 | ||
| Taiwan | Success | Vegetable | 1999 |
| Thailand | Success | Vegetable | 1998 |
| Tunisia | Vegetables, Citrus | 1998 | |
| Turkey | - | Grapes | 1998 |
| U.S. | Tracer, Conserve*
SpinTor*, Success |
Cotton
Turf & Ornamentals |
1997
1997 |
| Brassica Crops | 1998 | ||
| Apple, Tomato | 1998 | ||
| Tobacco | 1998 | ||
| Leafy Vegetables | 1998 | ||
| Citrus, Almonds | 1998 | ||
| Cucurbits, Legumes | 1998 | ||
| Stone Fruit, Potatoes | 1999 | ||
| Cereal Grains | 1999 | ||
| Minor Crops | 1999+ | ||
| UAE | _ | Vegetable | 1998 |
| Uruguay | Success | Citrus | 1998 |
| Venezuela | Tracer | Corn | 1998 |
*Trademark of Dow AgroSciences LLC
** Registration Dates are estimates
The favorable mammalian and environmental profile, insect selectivity and IPM fit, unique mode of action and resistance management properties, and outstanding efficacy are resulting in rapid registrations and adoption by growers around the world documenting the value to agriculture.
Literature Cited
- Anonymous. 1996. Spinosad technical guide. DowElanco (now Dow AgroSciences LLC), 25 pp.
- Carson, W. G. and J. T. Trumble. 1997. Effect of insecticides on celery insects, 1995. Arthropod Management Tests: 1997, vol. 22:117.
- Fouche, C., M. Canevari and D. Cutter. 1998. Evaluation of insecticides for control of leafminers on lima beans, 1997. Arthropod Management Tests. 23:74-75.
- Kerns, D. L. 1996. Control of lepidopterous larvae and leafminers in lettuce, 1995. Arthropod Management Tests. 21:117-118.
- Kirst, H. A., K. H. Michel, J. S. Mynderse, E. H. Chao, R. C. Yao, W. M. Nakatsukasa, L. D. Boeck, J.
- Occlowitz, J. W. Paschel, J. B. Deeter and G. D. Thompson. 1992. Discovery, isolation and structure elucidation of a family of structurally unique fermentation-derived tetracyclic macrolides. In: D. R. Baker, J. G. Fenyes and J. J. Steffens, Eds., Synthesis and chemistry of agrochemicals III. Am. Chem. Soc., Washington, D. C., pp. 214-225.
- Larson, L. L. 1997. Effects of adjuvants on the activity of Tracer™ 480SC on cotton in the laboratory, 1996. Arthropod Management Tests. 22:415-416.
- Linduska, J. J., M. Ross, D. Baumann and A. Parr. 1998. Foliar sprays to control ear-invading insects on sweet corn, 1997. Arthropod Management Tests: 23:95-96.
- Mayer, D. F. and J.D. Lunden. 1998. Research Reports: 72nd Annual Western Orchard Pest & Disease
- Management Conference. Oregon Extension Service. pp73-74.
- McLeod, P. 1998. Evaluation of insecticides for control of corn earworm on snap bean, 1997. Arthropod Management Tests. 23:75.
- Palumbo, J. C. 1997. Evaluation of selective insecticides for control of lepidopterous larvae in lettuce. Arthropod Management Tests. 22:136.
- Riley, D. G. 1998. Evaluation of insecticide treatments on cabbage, 1997. Arthropod Management Tests. 23:82.
- Salgado, V. L. 1997. The mode of action of spinosad and other insect control products. Down to Earth. 52(1), 35-44.
- Salgado, V.L. 1998. Studies on the mode of action of spinosad: Insect symptoms and physiology correlates. Pesticide Biochemistry and Physiology. 60(2):91-102.
- Salgado, V. L. , J.J. Sheets, G. B. Watson, A. L. Schmidt. 1998. Studies on the mode of action of spinosad: The internal effective concentration and the concentration dependence of neural excitation. Pesticide Biochemistry and Physiology. 60(2):103-110.
- Schoonover, J. R. and L. L. Larson. 1995. Laboratory activity of spinosad on non-target beneficial arthropods, 1994. Arthropod Management Tests. 20:357.
- Schuster, D. J. 1997. Management of insects on fresh market tomatoes, spring, 1996A. Arthropod Management Tests. 22:182.
- Sparks, T. C.; G. D Thompson,. H. A. Kirst,. M. B. Hertlein, J. S. Mynderse, J. R Turner,.T. V. Worden, (1998). Fermentation-Derived Insect Control Agents The Spinosyns. In: Methods in Biotechnology, Biopesticides: Use and Delivery. (eds. F. R. Hall and J. J. Menn). 5: 171-188. Humana Press. Totowa, NJ.
- Stansly, P. A. and J. M. Connor. 1998. Impact of insecticides alone and in rotation on tomato pinworm, leafminer and beneficial arthropods in staked tomato, 1997. Arthropod Management Tests: 23:162-165.
- Walgenbach, J. F. and C. R. Palmer. 1997. Control of lepidopterous insects on cabbage, 1996. Arthropod Management Tests. 22:113.
- Webb, S. E. 1998. Control of pickleworm on squash with selective insecticides, 1997. Arthropod Management Tests. 23:142-143.