Reproduced with permission from Valley Potato Grower© 1996 Red River Valley Potato Growers Association
Ted Radcliffe & Dave Ragsdale Department of Entomology, University of Minnesota Abdelaziz Lagnaoui World Bank, Washington, NY
Potato growers are well aware of the threat posed by Phytophthora infestans, the causal agent of potato late blight. With the appearance of the A2 mating type giving rise of metalaxyl (Ridomil®) resistant isolates, routine application of protective sprays has become increasingly critical to successful potato production. Growers are spraying fungicides more frequently and consequently the vines tend to remain green and vigorous late into the season. This association between greater use of late blight fungicides and increased difficulty of vine kill is obvious to growers. Most also recognize that this provides a situation favorable for green peach aphid, Myzus persicae. With higher green peach aphid numbers, there is greater risk of potato leafroll virus (PLRV) transmission and with healthy growing plants more likelihood of PLRV moving into the tubers. Fewer growers are aware of the remarkable direct impacts potato fungicides can have on green peach aphid. These effects are primarily the consequence of suppression of fungi (Family Entomophthoraceae) that cause mycoses (fungal infections) that kill this aphid.
Green peach aphid is far and away the most important vector of PLRV. In our region, spread of PLRV within the field is closely correlated with numbers of green peach aphids present on the crop. Movement of PLRV from sources outside the field by winged aphids appears to play a relatively minor role in the spread of PLRV. This is very different than potato virus Y (PVY) which is moved almost exclusively by winged aphids but of many different species.
Winged aphids invade potato in late June or early July. These early arrivals may be long distance migrants. There is no evidence that green peach aphid can successfully overwinter outdoors in our area. Insect natural enemies, i.e., generalist predators and parasitoids, can be very effective in holding green peach aphid numbers in check. Green peach aphid owes much of its pest status to human intervention. When insecticides are applied to control of Colorado potato beetle or other insect pests biological control agents are decimated. But, green peach aphid is resistant to most insecticides. Under such circumstances, green peach aphid populations can increase very rapidly, numbers can double every 2 to 3 days. Thus, green peach aphid tends to be a mid to late season pest and outbreaks are most commonly associated with intensive insecticide use. Most pyrethroid, phosphate, and organophosphate insecticides "flare" aphid numbers.
Potato entomologists have tended to consider entomopathogenic fungi of limited value in control of aphids. Reasons advanced for this presumed general ineffectiveness are: inadequate inoculum levels, infection being too dependent upon specific environmental conditions, and dissemination being too dependent upon the presence of uniformly distributed and abundant hosts. However, fungal diseases do end many aphid outbreaks and may contribute to holding subeconomic aphid populations in check.
The research reported here is not recent; it was done 10 years ago by Abdelaziz Lagnaoui, then a graduate student with Ted Radcliffe. However, these observations are of practical importance to potato growers, especially seed growers, and more so with the present intensive use of protective fungicidal sprays for control of metalaxyl-resistant potato late blight.
These experiments were conducted at the University of Minnesota Agricultural Experiment Station, Rosemount, Minnesota in 1985 and 1986. The data reported is from 1986, but 1985 results were similar. In 1986, a single experiment was conducted with insecticides applied as split-plot treatments to produce two densities of green peach aphid. Azinphosmethyl (Guthion®) was used to induce high green peach aphid densities; methoxychlor (Methoxychlor®) was used to induce moderate green peach aphid densities. Green peach aphid is highly resistant to both insecticides but azinphosmethyl has greater adverse impact on natural enemies.
Six fungicides were used: captafol (Difolatan®), chlorothalonil®), copper hydroxide (Kocide®), mancozeb (Dithane®), triphenyltin hydroxide (Du-Ter®) and metalaxyl (Ridomil). Each was applied at the upper rate labeled for use on potato. Control treatments without fungicide were included in each experiment.
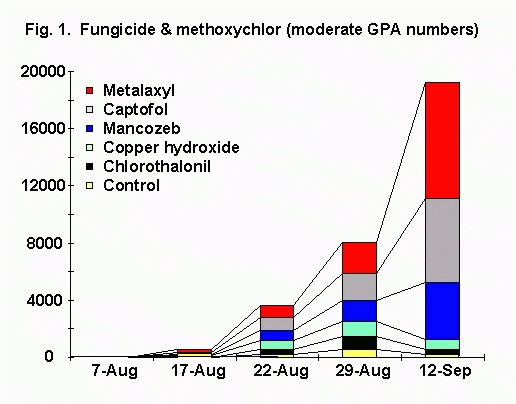
Aphid Populations. In plots sprayed with methoxychlor (Fig. 1), mean densities of green peach aphid across treatments ranged from 0 per leaf on 10 July to 92 per leaf on 12 September. On 12 September, all fungicidal treatments had significantly more aphids than did the control. From 29 August to 12 September, green peach aphid numbers in metalaxyl, captafol, and mancozeb treatments increased 179-277%, whereas in chlorothalonil, copper hydroxide, and the control, green peach aphid numbers decreased 37-69%.
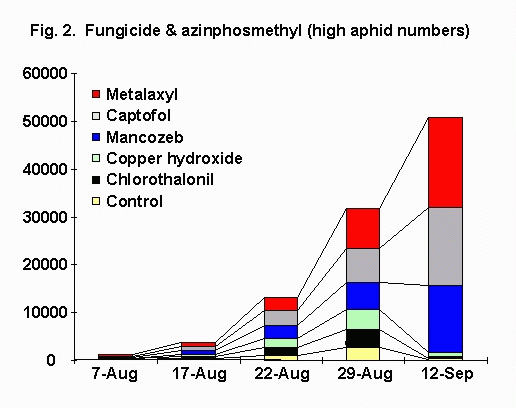
In plots sprayed with azinphosmethyl (Fig. 2), mean densities of green peach aphid densities across treatments ranged from 0 per leaf on 10 July to 149 per leaf on 12 September. On 12 September, metalaxyl, captafol, and mancozeb treatments had significantly more aphids than did the control. From 29 August to 12 September, green peach aphid numbers in metalaxyl, captafol, and mancozeb treatments increased 179-277%, whereas in chlorothalonil, copper hydroxide, and the control, green peach aphid numbers decreased 69-95%.
Incidence of Mycoses. On 29 August, the incidence of mycoses in plots sprayed with methoxychlor ranged from 2% (metalaxyl) to 8.7% (chlorothalonil) in fungicidal treatments and was 8.2% in the control (Fig. 3). Over the next two weeks, aphid densities and infection incidence increased greatly. On 12 September, infection incidence ranged from 5.6% (metalaxyl) to 22.1% (chlorothalonil) in fungicidal treatments and was 26.3% in the control.
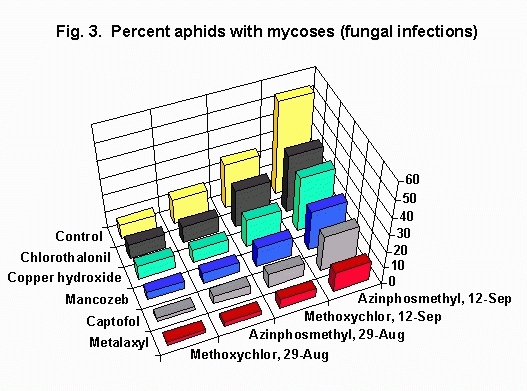
On 29 August, infection incidence in plots sprayed with azinphosmethyl, ranged from 2.5% (metalaxyl) to 9.6% (chlorothalonil) in fungicidal treatments and was 14.4% in the control (Table 3). On 12 September, infection incidence ranged from 11.9% (metalaxyl) to 33.8% (chlorothalonil) in fungicidal treatments and was 53.9% in the control.
We identified three species of fungi associated with green peach aphid. Pandora neoaphidis accounted for 67% of all mycoses, Entomophthora planchoniana 22%, and Conidiobulus. obscurus 8%. An unidentified species accounted for the remaining 3%. In the laboratory, pathogenicity to green peach aphid was greatest for E. planchoniana, intermediate for P. neoaphidis, and least for C. obscurus.
Toxicity of fungicides to entomopathogenic fungi: Effects on the germination of conidia and growth of mycelia of the three identified fungal pathogens were measured in laboratory bioassays. Germination of conidia was reduced significantly by all fungicides except chlorothalonil. Captafol, mancozeb and metalaxyl severely inhibited germination of conidia even at 0.1X rates of application. Growth of mycelia of were significantly inhibited by captafol, triphenyltin hydroxide, mancozeb, and metalaxyl. Azinphosmethyl and methoxychlor caused no inhibition of growth of either conidia or mycelia.
Fungal pathogenicity to aphids: Pathogenicities of primary spores of the three fungi to green peach aphid were measured in laboratory bioassays. Both in vivo and in vitro isolates were used. In vivo isolates were produced on site from cadavers of aphids recently killed in the field. In vitro isolates were from the USDA collection. Our results showed that fungi maintained on culture media (in vitro) were less infectious than those isolated from cadavers. Pathogenicity was greatest for E. planchoniana, intermediate for P. neoaphidis, and least for C. obscurus.
Toxicity of fungicides to aphids: We used dip tests to test fungicides for direct toxicity to green peach aphids. Most toxic were chlorothalonil, and copper hydroxide each of which caused 21% mortality at 48 hours exposure. Least toxic were captafol, mancozeb, and metalaxyl which caused mortality of 9, 12, and 15% mortality at 48 hours exposure.
Implications: Fungicides used to protect potato from foliar pathogens can be highly detrimental to entomophthoran fungi. Thus, the potential exists for upsetting biological control and triggering green peach aphid outbreaks when certain fungicides are used. We found that fungicides varied greatly in their effects on entomopathogenic fungi. This was reflected by greatly different levels of mycoses in the field and differences in effects on both conidia and mycelia in the laboratory. The result was that late season populations of aphids in field plots differed by more than 100 fold among fungicidal treatments. It is evident that careful selection of fungicides is a must if we wish to benefit from the natural control of green peach aphid afforded by entomophthoran fungi. This consideration is especially important in seed potato production because of the role of green peach aphid as a vector of potato viruses or when growing cultivars such as Russet Burbank which are susceptible to "net necrosis" (a tuber condition that can be caused by infection with potato leafroll virus).