William A. Overholt, A.J. Ngi-Song, C.O. Omwega, S.W. Kimani-Njogu, J. Mbapila, M.N. Sallam and V. Ofomata
International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya
Introduction

Many stemborers occur in Africa, but only a few species are economically important in maize, millet or sorghum. The noctuids, Busseola fusca (Fuller) and Sesamia calamistis Hampson, are widely distributed in sub-saharan Africa and reach damaging levels in some locations. B. fusca is a serious pest of maize at high elevations in East and southern Africa (Harris 1992). In West Africa, B. fusca is abundant in the dryer savana zone, particularly in areas where sorghum is grown (Harris, 1962). Sesamia calamistis occurs throughout sub-saharan Africa but is only a serious pest of cultivated cereals in West Africa (Bosque-Perez and Mareck 1990). Sesamia cretica Lederer is found in northeast Africa, the middle east and Mediterranean Europe and is considered to be a major pest of sorghum where it occurs (Tams and Bowden 1953). Coniesta ignefusalis Hampson (Pyralidae) is an important pest of millet in the Sahelian region of Africa (Nwanze 1991). Eldana saccharina (Pyralidae) is found throughout sub-saharan Africa and is considered a pest of maize and sugarcane in West Africa (Moyal 1988, Scheibelreiter 1980, Bosque-Perez and Mareck 1990). In Uganda, Girling (1978) reported that the pest status of E. saccharina changed radically in the early 1970s. Formerly, it was restricted to wild hosts, but then expanded its host range to include maize, sorghum and sugarcane. In South Africa, E. saccharina is considered to be a pest of sugarcane (Atkinson 1979). Chilo orichalcociliellus has been reported from coastal East Africa, Madagascar, Malawi and Nigeria (Mathez 1972, Delobel 1975a, Bleszynski 1970, Usua 1987), although the occurrence in Nigeria may have been based on a misidentification ( Meijerman and Ulenberg 1996). It was formerly considered to be an important pest in coastal Kenya and Madagascar (Mathez 1972, Warui and Kuria 1983, Delobel 1975a), but its pest status has changed with the establishment of the exotic stemborer, C. partellus. Chilo partellus is the only exotic stemborer in Africa (Overholt et al. 1994a) and is now found throughout East and southern Africa, where it is considered to be one of the most important stemborers in maize and sorghum.
The geographic distributions of the two most damaging cereal stemborers of maize and sorghum in East and southern Africa, B. fusca and C. partellus, are generally thought to be dependent on elevation (Ingram 1958, Nye 1960, Seshu Reddy 1983, Harris 1992). C. partellus reportedly occurs below about 1500 m, whereas B. fusca is found at elevations greater than 600 m. Sithole (1987) challenged this hypothesis and indicated that temperature, rainfall and humidity were the factors responsible for the distributions of these two stemborers, with temperature being the most important. He indicated that C. partellus was found in warmer regions and B. fusca in cooler areas. As temperature and elevation are highly correlated, both generalizations are probably true. Chilo orichalcociliellus Strand occurs in the coastal area of East Africa at elevations < 600 m (Nye 1960). S. calamistis is reportedly found in low, mid and high elevation maize and sorghum growing regions (Ingram 1958, Nye 1960).
Indigenous parasitoids of African stemborers have expanded their host ranges to include the exotic stemborer, but do not appear to effectively regulate densities at acceptable levels (Oloo and Ogedah 1990, Kfir 1992). Because of the economic importance of C. partellus, and its status as an introduced pest, it has been the target of three classical biological control attempts in Africa. The Commonwealth Institute of Biological Control (CIBC) imported nine species of parasitoids of C. partellus from India and released these in Uganda, Tanzania, and Kenya from 1968-1972 (CIBC 1968-72). In South Africa, 13 exotic parasitoids were introduced from 1977 to 1993 (Kfir 1994). No establishments were reported in either of the programmes.

A third attempt to introduce exotic parasitoids for control of C. partellus was initiated in Kenya in 1991 by the International Centre of Insect Physiology and Ecology (ICIPE) (Overholt 1993). This programme has focussed on the introduction of Cotesia flavipes Cameron (Hymenoptera: Braconidae), which was also released in the earlier programmes against C. partellus mentioned above. C. flavipes is native to the Indo-Australian region, but has been widely introduced against various stemborers in the neotropics, several Indian Ocean Islands, and also been redistributed within Asia (Overholt et al. 1994a). Cotesia flavipes was selected as the first candidate for introduction because of its history of success outside of Africa, and its importance as a parasitoid of stemborers in its aboriginal home (Overholt et al. 1994a). The C. flavipes released in Kenya originated from material collected in the Sindh region of Pakistan from C. partellus in maize by the International Institute of Biological Control (Omwega et al. 1995). The present paper reviews the studies that were conducted on C. flavipes at ICIPE prior to and after release in Kenya, and to provide evidence of its establishment in Kenya and in Tanzania.
Feeding Habits of Insect Predators

Many stemborers occur in Africa, but only a few species are economically important in maize, millet or sorghum. The noctuids, Busseola fusca (Fuller) and Sesamia calamistis Hampson, are widely distributed in sub-saharan Africa and reach damaging levels in some locations. B. fusca is a serious pest of maize at high elevations in East and southern Africa (Harris 1992). In West Africa, B. fusca is abundant in the dryer savana zone, particularly in areas where sorghum is grown (Harris, 1962). Sesamia calamistis occurs throughout sub-saharan Africa but is only a serious pest of cultivated cereals in West Africa (Bosque-Perez and Mareck 1990). Sesamia cretica Lederer is found in northeast Africa, the middle east and Mediterranean Europe and is considered to be a major pest of sorghum where it occurs (Tams and Bowden 1953). Coniesta ignefusalis Hampson (Pyralidae) is an important pest of millet in the Sahelian region of Africa (Nwanze 1991). Eldana saccharina (Pyralidae) is found throughout sub-saharan Africa and is considered a pest of maize and sugarcane in West Africa (Moyal 1988, Scheibelreiter 1980, Bosque-Perez and Mareck 1990). In Uganda, Girling (1978) reported that the pest status of E. saccharina changed radically in the early 1970s. Formerly, it was restricted to wild hosts, but then expanded its host range to include maize, sorghum and sugarcane. In South Africa, E. saccharina is considered to be a pest of sugarcane (Atkinson 1979). Chilo orichalcociliellus has been reported from coastal East Africa, Madagascar, Malawi and Nigeria (Mathez 1972, Delobel 1975a, Bleszynski 1970, Usua 1987), although the occurrence in Nigeria may have been based on a misidentification ( Meijerman and Ulenberg 1996). It was formerly considered to be an important pest in coastal Kenya and Madagascar (Mathez 1972, Warui and Kuria 1983, Delobel 1975a), but its pest status has changed with the establishment of the exotic stemborer, C. partellus. Chilo partellus is the only exotic stemborer in Africa (Overholt et al. 1994a) and is now found throughout East and southern Africa, where it is considered to be one of the most important stemborers in maize and sorghum.
The geographic distributions of the two most damaging cereal stemborers of maize and sorghum in East and southern Africa, B. fusca and C. partellus, are generally thought to be dependent on elevation (Ingram 1958, Nye 1960, Seshu Reddy 1983, Harris 1992). C. partellus reportedly occurs below about 1500 m, whereas B. fusca is found at elevations greater than 600 m. Sithole (1987) challenged this hypothesis and indicated that temperature, rainfall and humidity were the factors responsible for the distributions of these two stemborers, with temperature being the most important. He indicated that C. partellus was found in warmer regions and B. fusca in cooler areas. As temperature and elevation are highly correlated, both generalizations are probably true. Chilo orichalcociliellus Strand occurs in the coastal area of East Africa at elevations < 600 m (Nye 1960). S. calamistis is reportedly found in low, mid and high elevation maize and sorghum growing regions (Ingram 1958, Nye 1960).
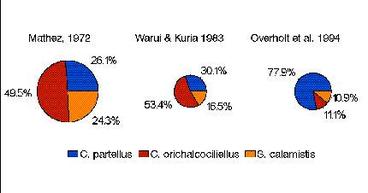
Results of recent surveys of stemborers in Kenya conducted from 1991 to present agree with the elevation-species abundance relationship described above (Overholt et al. 1994b, Khan unpublished). In the southern coastal area of Kenya (0-500 m), C. partellus was by far the most abundant species in maize, sorghum, and two wild grasses, Sorghum arundinaceum, and Panicum maximum, typically accounting for > 80% of the stemborers collected. Two indigenous species, C. orichalcociliellus and S. calamistis, also occurred. C. orichalcociliellus was the most abundant species in Pennisetum purpureum, a native grass grown as a forage crop. These results contrast with those found in earlier studies which indicated that C. orichalcociliellus was the predominant species, or equally abundant as C. partellus (Mathez 1972, Warui and Kuria 1983), although there is no evidence that total stemborer densities have changed (Overholt et al. 1994a). The apparent shift in the abundance of the two Chilo species during the recent past suggests that the exotic stemborer may be displacing the indigenous species. The effect of such a displacement on crop damage is unknown. However, recently completed studies at ICIPE have shown that C. partellus larvae consume more maize on a daily basis, and during the entire larval lifetime, than C. orichalcociliellus (Ofomata, unpublished). Thus, even if the total stemborer density in the coastal area of Kenya has not changed since the invasion of C. partellus, damage to maize and sorghum may be greater.
Host range and host finding behaviour of C. flavipes
In its aboriginal home, C. flavipes has been reported to parasitize several gramineous stemborers. In the neotropics, C. flavipes attacks several stemborers in the genus Diatraea. Thus, it appears that C. flavipes has a fairly wide host range. Because two or more stemborer species often occur sympatrically in Africa, it was important to determine the host range of C. flavipes prior to its release. Host range studies were also conducted on Cotesia sesamiae, an indigenous parasitiod which is closely related to C. flavipes and fills an ecologically similar niche. Laboratory studies revealed that C. partellus, C. orichalcociliellus, and S. calamistis were acceptable and suitable hosts for parasitization by C. flavipes and C. sesamiae (Ngi-Song et al. 1995) (Table 1). B. fusca and E. saccharina were aceptable for ovipostion, but no parasitoid progeny developed to maturity in either host. The inability of C. sesamiae to successfully develop in B. fusca was surprising, as this parasitoid has been recorded as the most common parasitoid of B. fusca in many countries in sub-saharan Africa (Mohyuddin and Greathead 1970, Kfir 1995). The laboratory colony of C. sesamiae used for our host range studies originated from material collected in coastal Kenya, where B. fusca does not occur. Recently, we have initiated a colony of C. sesamiae collected from B. fusca in western Kenya. Preliminary results indicate that approximately 83% of the B. fusca larvae exposed to this population of C. sesamiae are successfully parasitized (Ngi-Song unpublished). Thus, there is evidence of two biologically distinct populations of C. sesamiae; one that can develop in B. fusca, and one that cannot.
Table 1. Host suitability of Africa gramineous stemborers for Cotesia flavipes1.
| C. flavipes | C. sesamiae (coast) | |||
|---|---|---|---|---|
| Host species | %Parasitism | Progeny | %Parasitism | Progeny |
| Chilo partellus | 76.4 | 36.5 | 73.3 |
20.5 |
| Chilo orichalcociliellus | 63.2 | 32.8 | 58.3 | 22.9 |
| Sesamia calamistis | 42.2 | 34.0 | 76.7 | 35.3 |
| Busseola fusca | 0.0 | - | 0.0 | - |
| Eldana saccharina | 0.0 | - | 0.0 | - |
1Data for C. partellus, C. orichalcociliellus, S. calamistis and B. fusca: Ngi-Song et al. 1995. Data for E. saccharina: Overholt unpublished
The host finding behaviours of C. flavipes and C. sesamiae were investigated by examining the responses of the parasitoids to volatile odours from stemborers, plants and by-products of stemborer feeding. Both parasitoids responded more strongly to unwashed C. partellus larvae removed from maize stems than to larvae washed in distilled water after removal (Ngi-Song 1995), and in a dual choice test, C. flavipes responded more strongly to frass than to stemborers (Potting et al. 1995). Several grasses not infested with stemborers proved to be attractive C. flavipes and C. sesamiae, but infested plants provoked a stronger response (Ngi-Song et al. 1996). Plants infested with all the stemborer species tested (C. partellus, C. orichalcociliellus, S. calamistis and B. fusca) were attractive, and attraction was related to the number and size of the feeding stemborers (Ngi-Song et al. 1996). Infested host plants released a synomone that was attractive to parasitoids (Potting et al. 1995), and frass from all stemborer/host grass combinations examined proved to be highly attractive (Ngi-Song 1995). A slight difference in attraction to maize and sorghum was found between the two parasitoids. C. flavipes responded more strongly to maize, while C. sesamiae exhibited a preference for sorghum (Ngi-Song et al. 1996). In summary, both parasitoids were attracted to volatile odours emanating from stemborers in grasses, regardless of whether the stemborer was a suitable host. These results suggest that if C. flavipes were to be released in areas where suitable and unsuitable occurred sympatrically, the parasitoid population would suffer mortality in the unsuitable hosts.

Chilo partellus and C. orichalcociliellus populations are sustained during dry non-cropping periods through two mechanisms; aestivation as late instar larvae in crop refuse (Scheltes 1978), and as non-aestivating larvae in wild grasses (Delobel 1975b). Studies on the host finding ability of the two parasitoids for aestivating and non-aestivating larvae revealed that neither parasitoid was capable of locating aestivating larvae in dried maize stems (Mbapila 1997). The cues necessary for host finding were apparently absent in the aestivating larvae/senescent plant combination. In an artificial laboratory setting, C. flavipes would parasitize aestivating larvae when the host and parasitoid were placed in close proximity. The developmental times of parasitoids in aestivating and non-aestivating larvae were not different, suggesting that the endocrine environment of the host did not induce diapause in C. flavipes (Mbapila, 1997). The results of these studies suggest that C. flavipes must locate non-aestivating larvae in wild host plants in order to survive during non-cropping seasons.
Biosystematics of the Cotesia flavipes species complex
Prior to release, it was essential to be able to reliably distinguish C. flavipes from C. sesamiae. Additionally, a third species, C. chilonis, was included in the study as it was considered to be closely related to C. flavipes and C. chilonis (Polaszek and Walker 1991), and has also been the subject of introductions into Africa (Bordat 1983, Kfir 1994). Previous work had indicated that the three species could be reliably separated into two morpho-species by the form of the male genitalia, with C. sesamiae and C. chilonis being indistinguishable (Polaszek and Walker 1991). Morphological and biochemical analyses revealed several additional characters on males and females that could be used to accurately separate the three species. For example, the shape of the scuto-scutellar-sulcus on the pronotum separated males and females of C. chilonis from the other two species, and the rugosity of propodeum separated C. sesamiae from the other species. Additionally, material from Mauitius that was putatively C. flavipes, had male genitalia that was distinct from other populations of C. flavipes examined, and also differed from the C. sesamiae/C. chilonis male genitalia (Kimani 1995). This finding suggests that the population in Mauritius could be a distinct taxon, although interbreeding tests with other C. flavipes populations need to be conducted before reaching a final conclusion.
In addition to the taxonomic separation of the species, mating experiments revealed that all possible combinations of males and females of the three species would interbreed in the laboratory. However, the only crosses which produced female offspring were the monogametic crosses, and the cross between C. sesamiae males and female C. chilonis females. Crosses between C. flavipes and C. sesamiae did not produce female progeny. Investigations on male-female attraction demonstrated that C. flavipes males were attracted to conspecfic females, but not to females of the other two species, suggesting that opportunities for interbreeding in nature would be rare (Kimani and Overholt 1995).
Competition between C. flavipes and C. sesamiae
Intrinsic and extrinsic competition between C. flavipes and C. sesamiae were investigated in various laboratory and field studies. When C. partellus was stung by both parasitoids, C. flavipes emerged in nearly all cases, regardless of which parasitoid had stung the host first (Sallam, unpublished). Laboratory life tables were constructed for the two parasitoids at several temperatures using C. partellus as a host. At all temperatures, the intrinsic rate of population increase of C. flavipes was higher than C. sesamiae, suggesting that the exotic parasitoid was capable of responding more rapidly to changes in host density (Mbapila 1994). Functional response experiments in large field cages demonstrated that C. flavipes successfully attacked more hosts at all host densities examined (Sallam unpublished). The results of the competition studies suggest that C. flavipes is a superior parasitoid when C. partellus is the host. As these two parsasitoids fill an ecologically very similar, if not homologous, niche, it is anticipated that local displacement may occur in areas where C. partellus is the predominant stemborer. However, similar studies on competition need to be conducted using indigenous stemborers as hosts.
Release and establishment
In the long rainy season of 1993 (March-July), C. flavipes was released at three locations in the coastal area of Kenya over a period of 6-8 weeks (Overholt et al. 1994c). The number of female parasitoids liberated at each site was estimated to be between 18,100 and 24,200. Parasitoids were released both as adults and as cocoons. Release of cocoons was considered to be a preferred method as it maximized the effective lifespan of the adults in the field. Adult C. flavipes live only a few days (Wiedenmann et al. 1992), whereas the cocoon stage lasts 5-6 days (Kajita and Drake 1969). Thus, if adults which emerged in the laboratory were released, a significant portion of their adult life span could have passed before liberation, particularly if release sites were distant from the laboratory. Additionally, it was logistically simpler to release cocoons as the timing of visits to the field wsa less critical than with adults. The cocoons could be placed in the field at anytime during the 5-6 day window of opportunity. The protect the cocoons from predators and rainfall, they were placed in a 'release station' (Overholt et al. 1994c).
C. flavipes successfully located and parasitized stemborers at the three sites during the season of release (Overholt et al. 1994c). All three stemborers species found at the coast, C. partellus, C. orichalcociliellus, and S. calamistis, were parasitized (Overholt, unpublished data), confirming the results of previous laboratory studies (Ngi-Song et al. 1995, Ngi-Song et al. 1996). C. flavipes was also recovered during sampling in the short rainy season (Oct-Dec) in 1993 at the release sites when 19 C. partellus and one S. calamistis were found to be parasitized (Overholt unpublished).
C. flavipes was released at a fourth site during the dry season of 1994 in an area where the vegetation was predominated by the wild grass, Sorghum arundinaceum. This release was conducted to determine whether C. flavipes could colonize the wild sorghum habitat and then move into a maize agroecosystem during the following rainy season. Approximately 6000 females were released over a period of 4 weeks. A maize field was planted adjacent to the wild sorghum once the long rains began and both plants were sampled throughout the rainy season. Approximately 2.2 and 5.5% of C. partellus medium and large-sized larvae were parasitized by C. flavipes in wild sorghum and maize, respectively (Overholt unpublished).
In addition to sampling at the wild sorghum site, seven other maize fields were sampled during the long rains of 1994. C. flavipes was recovered at only one site. During the following short rains, 21 sites were sampled, and C. flavipes was found at seven. Seasonal parasitism at the seven recovery sites was and ranged from 0.3% to 3.0%. In the 1995 long rains, 29 sites were sampled at the coast. C. flavipes was found at 4 of the sites, but parasitism was low ranging between 0.05% and 1.0% (Overholt unpublished). Data from the 1996 long rains cropping season has not yet been completely analyzed, but the number of recoveries of C. flavipes increased dramatically and it was the most abundant parasitoid at 5 of the 11 sites it was recovered. The recovery of stemborers parasitized by C. flavipes three years after the release provides clear evidence that the exotic parasitoid is firmly established in the coastal area of Kenya. Furthermore, it had spread from the original release sites to other locations.
In May 1994, C. flavipes was collected from unidentified stemborers in the South Nyanza District which borders Lake Victoria in southwestern Kenya. This was quite surprising as no releases had been made in this area, and it is over 600 km from the coastal release sites. A more thorough survey was conducted in the area in June/July 1994 and C. flavipes emerged from C. partellus collected at seven locations in the same area (Omwega et al. 1995). In July 1995, a survey was conducted in northern and central Tanzania. C. flavipes was found at two locations, Tarime and Magu, which are both near Lake Victoria in the area bordering southwestern Kenya. Parasitism of C. partellus larvae was 44.4 and 62.7% at Tarime and Magu, respectively (Omwega unpublished). There are three possibilities that could explain the establishment in the Lake Victoria area; 1) C. flavipes established from releases made by CIBC in Uganda and Tanzania from 1968-72, 2) C. flavipes moved from the coastal area where it was released in 1993 and 1994, or 3) C. flavipes escaped from a laboratory colony that was maintained by ICIPE at Mbita Point Field Station in southwestern Kenya in 1991. Based on evidence from surveys conducted prior to 1994 and on electrophoretic evidence, it was concluded that the most likely possibility was that C. flavipes escapted from a colony maintained at Mbita Point in 1991 (Omwega et al. 1995).
Impact on stemborer populations
The levels of parasitism in the coastal area of Kenya are still quite low, but appear to be increasing with time. In Madagascar where C. flavipes was released against Chilo sacchariphagus in sugarcane, maximum levels of parasitism (60%) were not reached until 6 years after the releases (Betbeder-Matibet and Malinge 1967). In Barbados, where C. flavipes was released in 1966 against Diatraea saccharalis in sugarcane, it was not recovered for more than one year after the releases in spite of intensive surveys, but then parsaitism rose steadily during the next few years (Alam et al. 1971). During colonization, dispersal has a counteracting influence on increases in density. Natural selection may also depress initial population increase. The larger the geographic expanse of suitable habitat, the longer it will take the colonizing insect to reach a characteristic density. There is some evidence of this occuring with C. flavipes in East Africa. If our hypothesis is correct that the establishment in the Lake Victoria region resulted from parasitoids that escaped in 1991, then C. flavipes has had two years longer to colonize this area than the coast. Parasitism at Tarime and Magu were indeed much higher than has been found at the coast, although the results at these sites were based on a single sampling occasion at the end of the maize cropping season. Intensive surveys throughout the year are now being conducted at 3 locations in southwestern Kenya to more accurately measure stemborer mortality caused by C. flavipes.
References Cited
- Atkinson P. R. (1979) Distribution and natural hosts of Eldana saccharina Walker in Natal, its oviposition sites and feeding patterns. Proceedings of the South African Sugar Technologists' Association 53, 111-115.
- Alam M. M., Bennett F. D. and Carl K. P. (1971) Biological control of Diatraea saccharalis (F.) in Barbados by Apanteles flavipes Cam. and Lixophaga diatraeae T. T. Entomophaga 16, 151-158.
- Betbeder-Matibet M and Malinge P. (1967) Un succes de la lutte biologique: controle de Proceras sacchariphagus Boj. Borer ponctue de la canne a sucre a Madagascar par un parasite introduit: Apanteles flavipes Cam. Agronomie Tropical 22, 1196-1220.
- Bleszynski S. (1970) A revision of the world species of Chilo Zinchen (Lepidoptera: Pyralidae). Bulletin of the British Museum of Natural History, Entomology 25, 101-195.
- Bordat D. (1983) Mise au point de l'elevage de masse d'Apanteles chilonis Matsumura, 1912 et d'Apanteles flavipes (Cameron, 1891) (Hymenopteres Braconidae) sur trois lepidopteres pyralidae foreurs des graminees (Chilo zacconius Bleszynski, 1970; Chilo partellus (Swinhoe, 1884) et Diatraea saccharalis (Fabricius, 1794)) dans un objectif de lutte biologique. These. Universite des Sciences et Techniques du Langeudoc, Montpellier, France. 184 p.
- Bosque-Perez N. A. and Mareck J. H. (1990) Distribution and composition of Lepidopterous maize borers in souther Nigeria. Bulletin of Entomological Research 80, 363-368.
- CIBC (1968-72) Annual Reports of the Commonwealth Institute of Biological Control.
- Delobel A. (1975a) Chilo orichalcociliellus Strand (Lepidoptera: Pyralidae), foreur des tiges du sorgho et du mais a Madagascar II. Premieres donnees biologiques. ORSTOM Series Biologie 10, 11-16.
- Delobel, A. (1975b) Une population hivernante de Chilo partellus (Lepidoptera Pyralidae) sur la cote ouest de Madagascar. ORSTOM Series Biologie 10: 17-23.
- FAO. 1995. FAO yearbook 1994 production. Food and agricultural organization of the United Nations. Rome.
- Girling D. J. (1978) The distribution and biology of Eldana saccharina Walker (Lepidoptera: Pyralidae) and its relationshiop to other stemborers in Uganda. Bulletin of Entomological Research 68, 471-488.
- Gupta B. D. (1953) Resume of work done under the insect pest scheme during 1946-47 to 1950-51. Indian Centre Sugarcane Commercialization, New Delhi 111 pp.
- Harris K. M. (1962) Lepidopterous stem borers of cereals in Nigeria. Bulletin of Entomological Research 53, 139-171
- Harris K. M. and Nwanze K. F. (1992) Busseola fusca (Fuller), the African maize stalk borer: a handbook of information. Information bulletin 33. ICRSIAT Patancheru, India and CABI Oxon U.K. 84 p.
- Ingram W. R. (1958) The lepidopterous stalk-borers associated with Gramineae in Uganda. Bulletin of Entomological Research 49, 367-383.
- Kajita H. and Drake E. F. (1969) Biology of Apanteles chilonis and A. flavipes (Hymenoptera: Braconidae), parasites of Chilo suppressalis. Mushi 42: 163-179.
- Kfir R. (1992) Seasonal abundance of the stem borer Chilo partellus (Lepidoptera: Pyralidae) and its parasites on summer grain crops. Journal of Economic Entomology 85, 518-529.
- Kfir R. (1994) Attempts at biological control of the stem borer Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) in South Africa. African entomology 2(1): 67-68.
- Kfir, R. (1995) Parasitoids of the African stem borer, Busseola fusca (Lepidoptera: Noctuidae), in South Africa. Bull. Entomol. Res. 85: 369-377.
- Kimani S. W. and Overholt W. A. (1995). Biosystematics of the Cotesia flavipes complex (Hymenoptera: Braconidae): interspecific hybridization, sex pheromone and mating behaviour studies. Bulletin of Entomological Research 85: 379-386.
- Kimani S. W. (1995) Biosystematics of the Cotesia flavipes complex (Hym: Braconidae), parasitoids of gramineous stemborers. PhD Thesis. University of Nairobi, Kenya. 191 p.
- Mathez F. C. (1972) Chilo partellus Swinh., C. orichalcociliella Strand (Lep., Crambidae and Sesamia calamistis Hmps. (Lep., Noctuidae) on maize in the Coast Province, Kenya. Mitteilungender der Schweizerischen Entomologischen Gesellschaft 45, 267-289.
- Mbapila J. (1994) Laboratory life tables of Cotesia spp. reared at difference temperatures on Chilo partellus. pg. 31 In 1994 ICIPE Annual Report. ICIPE Science Press. 199 p.
- Mbapila, J. and Overholt, W. A. 1997. Oviposition, development and searching behaviour of Cotesia flavipes Cameron and Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae) in relation to aestivating and non-aestivating Chilo spp. (Swinhoe) (Lepidoptera: Pyralidae) in larvae in maize. African Entomology 5: 000-000.
- Meijerman L. and Ulenberg S. A. (1996). Identification of African stemborer larvae (Lepidoptera: Noctuidae, Pyralidae) based on morphology. Bull. Entomol. Res. 86: 567-578.
- Mohyuddin A. I. and Greathead D. J. (1970) An annotated list of the parasites of graminaceous stem borers in East Africa, with a discussion of their potential in biological control. Entomophaga 15, 241-274.
- Moyal P. 1988. Crop losses due to insects in the savannah area of Ivory Coast: a review. Tropical pest management 34(4): 455-459.
- Ngi-Song A. J. (1995) Parasitization of selected African stemborers by Cotesia flavipes Cameron and Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae), with emphasis on host selection and host suitability. PhD Thesis. University of Ghana, Legon. 198 p.
- Ngi-Song A. J., Overholt W. A. and Ayertey J. A. (1995) Suitability of African gramineous stemborers for development of Cotesia flavipes and C. sesamiae (Hymenoptera: Braconidae). Environmental Entomology 24(4): 978-984.
- Ngi-Song A. J., Overholt W. A., Njagi P. G. N., Dicke M., Ayertey J. A. and Lwande W. (1996) Volatile infochemicals used in host and host habitat location by Cotesia flavipes Cameron and Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae), larval parasitoids of stemborers on graminae. Journal of Chemical Ecology 22(2): 307-323.
- Nwanze K. F. (1991) Components for the management of two insect pests of pearl millet in Sahelian West Africa. Insect Sci. Applic. 12: 673-678.
- Nye I. W. B. (1960) The insect pests of graminaceous crops in East Africa. Colonial Research Study 31. London. Her Majesty's Stationary Office. 48 pp.
- Oloo, G. W. and Ogedah K. (1990) The incidence of Chilo partellus (Swinh.) (Pyralidae) and the contribution of natural enemies to its mortality under intercropping system in Kenya. Tropical Pest Management 36(3): 244-248.
- Omwega C. O., Kimani S. W., Overholt W. A and Ogol C. K. P. O. (1995) Evidence of the establishment of Cotesia flavipes (Hymenoptera: Bracondiae) in continental Africa. Bulletin of Entomological Research 85: 525-530.
- Overholt W. A. (1993) Release of beneficial insects in Kenya. Discovery and Innovation 5(3): 199-200.
- Overholt W. A., Ngi-Song A. J., Kimani S. W, Mbapila J., Lammers P. M. and. Kioko E. (1994a) Ecological considerations of the introduction of Cotesia flavipes Cameron (Hymenoptera: Braconidae) for biological control of Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae), in Africa. Biocontrol news and information 15(2): 19N-24N.
- Overholt W. A., Ogedah K. and Lammers P. M. (1994b) Distribution and sampling of Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) in maize and sorghum at the Kenya Coast. Bulletin of Entomological Research 84: 367-378.
- Overholt W. A., Ochieng J. O., Lammers P. and Ogedah K. (1994c) Rearing and field release methods for Cotesia flavipes Cameron (Hymenoptera: Braconidae), a parasitoid of tropical gramineous stem borers. Insect Sci. Appl. 15(3): 253-259.
- Polaszek A. and Walker A. K. (1991) The Cotesia flavipes species complex: parasitoids of cereal stemborers in the tropics. Redia 74, 335-341.
- Potting R. P. J., Vet L. E. M. and Dicke M. (1995) Host microhabitat location by stem-borer parasitoid Cotesia flavipes: the role of herbivore volatiles and locally and systematically induced plant volatiles. Journal of Chemical Ecology 21(5): 525-539.
- Scheibelreiter G. K (1980) Sugar-cane stem borers (Lepidoptera: Pyralidae and Noctuidae) in Ghana. Zeitshrift fur Angewandte Entomologie 89, 87-99.
- Scheltes P. (1978) Ecological and physiological aspects of aestivation-diapause in the larvae of the two pyralid stalk borers of maize in Kenya. PhD Thesis. Landbouwhogeschool, Wageningen.
- Senthamizhselvan M. and Muthukrishnan J. (1989) Bioenergetics of Apanteles flavipes Cameron (Hymenoptera: Braconidae), a parasitoid of Porthesia scintillans Walker (Lepidoptera: Lymantriidae). Insect Sci. Appl. 10(3): 295-299.
- Seshu Reddy K. V. (1983) Sorghum stem borers in eastern Africa. Insect Science and its Application 4, 33-39.
- Sithole S. Z. (1987) Maize insect pests in Zimbabwe. pp. 286-288 In Toward Insect Resistant Maize for the Third World. Proceedings of the International Symposium on Methodologies for Developing Host Plant Resistance to Maize Insects. Mexico, D. F. Centro Internacional de Majoramiento de Maiz y Trigo.
- Tams W. H. T. (1932) New species of African Heterocera. Entomologist 65, 1241-49.
- Tams W. H. T. and Bowden J. (1953) A revision of the African species of Sesamia Guenee and related genera (Agrotidae - Lepidoptera). Bulletin of Entomological Research 43, 645-678.
- Usua E. J. 1987. Descriptions of the larvae and pupae of some important lepidopterous stemborers of cereals. Entomological Society of Nigeria, Occasional Publication 29: 1-29.
- Van Hamburg H. (1979) The grain-sorghum stalk-borer, Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae): seasonal changes in adult populations in grain sorghum in the Transvaal. Journal of the Entomological Society of Southern Africa 42: 1-9.
- Warui C. M. and Kuria J. N. (1983) Population incidence and the control of maize stalk-borers Chilo partellus (Swinh.) and Chilo orichalcociliellus Stand and Sesamia calamistis Hmps. in Coast Province, Kenya. Insect Science and its Application 4, 11-18.
- Wiedenmann R. N., Smith J. W. Jr. and Darnell P. O. (1992) Laboratory rearing and biology of the parasite Cotesia flavipes (Hymemoptera: Braconidae) using Diatraea saccharalis (Lepidoptera: Pyralidae) as a host. Environmental Entomology 21, 1160-1167.
- Youdeowi, A. 1989. Major arthropod pests of food and industrial crops of Africa and their economic importance. In: Yanninek, J. S. and H. R. Herren (eds.) Biological control: a sustainable solution to crop pest problems in Africa. Ibadan, Nigeria. International Institute of Tropical Agriculture. pp. 51-60.
- Youm O., Gilstrap F. E. and Browning H. W. (1990) Parasitism of stem borers (Lepidoptera: Pyralidae) associated with corn and sorghum in the lower Rio Grande Valley of Texas. Journal of Eco nomic Entomology 83(1): 84-88.