James H. Tsai
Fort Lauderdale Research and Education Center
University of Florida, IFAS
Bryce W. Falk
Department of Plant Pathology
University of California, Davis, CA 95616
MAIZE (Zea mays L.) is one of the major cereal crops, it ranks third in production following wheat and rice with an average of 380 million tons produced annually on 120 million ha by 53 countries. It is the world's most widely grown crop in almost all tropical areas of the world including tropical highlands over 3000 m in altitude, to temperate areas as far north as the 65th latitude. Because of different ecological conditions exist between the temperate areas and the tropics, the insect vectors and their disease agents also are different under these different conditions. To date at least five viral and two mollicute plant pathogens affecting maize are vectored by the homopteran insects (aphids, delphacid planthopper and leafhoppers) in the hot and humid tropics. They include maize dwarf mosaic potyvirus (A and B), maize stripe tenuivirus, maize rayado fino marafivirus, maize mosaic nucleorhabdovirus, corn stunt spiroplasma and maize bushy stunt phytoplasma. This chapter is to focus on the homopteran vectors and their disease agents.
I. Aphid transmitted maize dwarf mosaic potyvirus (MDMV):
Maize dwarf mosaic is one of the most important widely distributed virus diseases of corn (Zea mays L.) in the temperate regions of the world and is especially a concern to seed producers. It is found in 37 states of the continental U.S.A. and Hawaii. This disease caused severe yield losses in the learly 1960's particularly in dent corn (76). However, the crop losses varied greatly depending on the susceptibility of the corn genotype, virus strains, plant age and environmental factors. Maize dwarf mosaic virus (MDMV) was named by Williams and Alexander (76) and is closely related to sugarcane mosaic virus (SCMV) which has at least 13 strains. In 1966 MacKenzie and associates (47) desbribed MDMV strains A and B on the basis that strain B does not infect Johnsongrass [Sorghum halepense (L.) Pers.]. Both MDMV strains A and B were reported from Florida in 1978 and 1979 (74). This disease has not been reported as a serious disease in the Tropics and Subtropics. This is mainly due to limited distribution of the aphid vectors.
1. Symptomatology and Host Range

Considerable variations of mosaic pattern may be produced by MDMV in corn. During initial symptom development, ligh and dark green mottles are evident on leaves. As the disease progresses, the alternating light and dark green mottles increase in intensity to form mosaics, flecks and rings on leaves (Fig. 1).
The mosaic pattern usually begins at the leaf base and may be irregular and diffuse. Mosaics produced by MDMV-A are often limited to the interveinal areas and so form stripes. Mosaic may also be evident on leaf sheaths and flag leaves of the ears. Plant stunting and poor ear fill may be associated with the mosaic symptoms.
The host range of MDMV is limited to the grass family. Of 66 grass genera tested from the U.S.A., 44 contained one or more susceptible species. Nearly 250 grass sepecies including species of Cynodon, Paspalum, and Pennisetum have been reported as hosts of MDMV; 243 species are susceptible to both MDMV-A and B, 38 grasses are susceptible to MDMV-B (64).
2. Properties of MDMV
MDMV particles are flexuous rods, typical of the potyvirus group measuring about 750 nm in length and 12-15 nm in diameter (74). Pinwheel inclusion bodies in infected tissue are often evident. MDMV-A causes 'Atlas' sorghum [Sorghum biocolor (L.) Moench]. It does not infect wheat. In contrast, MDMV-B infects corn, causing local lesions, and may become systemic on 'Atlas' sorghum and other sorghum cultivars. Strain B does not infect Johnsongrass or wheat but does infect St. Augustinegrass [Stenotaphrum secundatum (Walt.) Kuntze] in south Florida (74) and other species of Stenotaphrum.
3. Transmission MDMV
Seed transmission of MDMV (up to 0.5%) results in a hgh disease incidence in maturing plants (9). MDMV is also readily transmissible by aphids in a nonpersistent manner which means that both virus acquisition and inoculation by aphids can occur in a few seconds. At least 25 species of aphids have been reported to be vectors (42). The transmission efficiency varies greatly depending upon aphid species, environmental conditions, virus strains and host plants. The virus can survive in perennial grasses or in the seed of annual or perennial grasses which represent important sources for both MDMV and the aphids that transmit it. Long distance movement of the viruliferous aphids in low level wind jet streams can also be an important factor in virus spread. The aphid species known to be efficient vectors of MDMV are: the green bug, Schizaphis graminum (Rondani), the corn root aphid, Aphis maidiradicis Forbes, the cowpea aphid, A. craccivora Koch, the bean aphid, A. fabae Scopoli, the melon aphid, A. gossypii Glover, the boat gall aphid, Hyalopterus atriplicis (L.), the pea aphid, Acyrthosiphon pisum (Harris), the green peach aphid, Myzus persicae (Sulzer), the English grain aphid, Macrosiphum avenae (F), the blue grass aphid, the corn leaf aphid, Rhopalomyzus poae (Gillette) and Rhopalosiphum padi (L.) (42).
II. Corn delphacid transmitted maize stripe tenuivirus (MStV):
Maize stripe disease was first described in 1936 in E. Africa by Storey who recognized two types of symptoms, one with narrow yellow stripes on the leaves, the other with broad stripes (65). Kulkarni (43) demonstrated that two symptoms of maize stripe were associated with two distinct pathogens and were transmitted persistently by the corn delphacid, Peregrinus maidis (Ashmead). Later Bock et al. (8) proved that the narrow yellow stripe was caused by a rhabdovirus. To date maize stripe has been reported from Venezuela, Florida the Philippines, Mauritius, Australia, Peru and Taiwan (75).
1. Symptomatology and Host Range:
Initial symptoms on the inoculated plants are fine chlorotic stipplings between the veins which later develop into continuous chlorotic stripes of varying width and intensity (68)(Fig. 2A) , often with a 'brushed out' appearance (40) towards the tips of stripes (Fig. 2B). Young plants at the 4 to 5-leaf stage inoculated with MStV often exhibit complete chlorosis on the emerging whorl leaf, and the center leaf usually remains folded and bent (68). The host range of MStV includes Zea spp., and several Sorghum spp. as well as Rottboellia exaltata. This virus can infect barley, rye, oats, wheat and triticale under experimental conditions, (17,75).


2. Properties of MStV Nucleoproteins:
MStV is a member of newly recognized tenuivirus group which includes rice stripe virus (RSV), rice joja blanca virus (RHBV), rice grassy stunt virus (RGSV) and European wheat striate mosaic virus (EWSMV). This group exhibits several unique properties different from other characterized RNA plant viruses (31). Thin, filamentous, sometime circular infectious nucleoprotein particles have been associated with tenuivirus infected plants (15,75). The nucleoprotein particles are composed of a ca. 35,000 Mr nucleocapsid protein and 4-5 species of RNA (15,25,67). When analyzed by denaturing gel electrophoresis, the 5 RNAs have molecular weights of 0.52, 0.78, 0.81, 1.18 and 3.01 x 106. The complete nucleotide sequences of MStV RNA2, RNA3, RNA4 and RNA5 have been determined as 3337 nt, 2357 nt, 2227 nt and 1317 nt in size, respectively (22,25,29,75). The 16,000 Mr (16K) protein which has been referred to as the NCP is found abundanttly in MStV-infected plants (28) and it can readily be found in sap from infected plants as crystals by phase-contrast light microscopy and the crystals react with antiserum to MStV NCP in immunofluorescence microscopy (12). The NCP is found as large aggregates forming filamentous electron opaque inclusion bodies (4). Recently study showed that fibrous intracellar inclusins can be readily found in paradermal sections of the leaf sheath of MStV infected maize (59). The nucleotide sequence of MStV NCP gene has also been determined.
Antisera to the 32,000 Mr (32 K) capsid and 16,500 Mr (16 K) NCP have been used for immunological analyses of extracts from MStV-infected maize plants and from inoculative P. maidis. The antiserum against the noncapsid protein was found to be very useful for detecting MStV infections in plants by indirect ELISA (28,75). Other MStV-infected hosts such as rye (Secale cereale), itchgrass (Rottboellia exaltata) and oats (Avena sativa) were detected satisfactorily by indirect ELISA (23,75).
The 32 K protein was easily detected in extracts of both MStV-infected plants and inoculative P. maidis by ELISA and by immunological analysis of 'Western' blots (28). The 32 K protein was detected only in individual P. maidis that also transmitted MStV to plants. The 16 K protein was only detected in MStV-infected plant hosts but not from extracts of groups or of individual MStV inoculative P. maidis (17,75).
There are several other planthopper-borne viruses of gramineae which are related to MStV. One of these, rice stripe virus (RSV) from Taiwan and Japan, was reported to be composed of a 9-11 and 3-8 nm nucleoprotein, respectively (17,67). RSV also has been shown to be serologically related to MStV (17) based on tests using antisera to their capsid proteins, however, MStV noncapsid protein antiserum did not react with RSV infected rice (34). Indirect ELISA and one dimensional peptide-mapping analysis on two other planthopper-borne viruses of gramineae, rice hoja blanca virus (RHBV) and Echinochloa hoja blanca virus (EHBV) in comparison with MStV showed that the MStV noncapsid and capsid proteins were different from those corresponding proteins of RHBV and EHBV (28).
3. Transmission Characteristics of MStV:

MStV is transmitted by P. maidis in a persistent-propagative manner. Nymphs of P. maidis transmitted MStV with ca. twice the efficiency after a 24-, 48-, 68-, 96-, and 192 hr acquisition access period (AAP) as did adults. Macropterous adults were slightly more efficient transmitters than brachypterous adults (Fig. 3).
The minimum AAP of MStV by nymphs was 4 hr. The minimum incubation period (IP) in both nymphs and adults was 4 to 5 days. The average retention of MStV by 2nd and 3rd instars was 13.7, 13.2, and 15.8 days after 48, 72, and 98 hr AAP as compared to 3.7, 5.5, and 6.5 days retention by adults. MStV could also be transmitted by sap injection, hemolymph injection and transovarial passages (69). The rate of transovarial transmission was reported as high as 33.3% (69) and 59% (32). In a time course study, no 32 K protein was detected in P. maidis until 8 days after the beginning of a 5-day AAP on MStV-infected plants. The percentage of MStV-positive P. maidis increased overtime indicating multiplication of MStV in P. maidis (28). These data suggest that MStV is propagative in P. maidis.
4. Biology of P. maidis:
P. maidis is pantropical species and has been recorded from most tropical regions (70). In general, there are 5 stadia in the nymphal stage. however, the number of stadia varied depending on the temperatures. The nymphal development time and adult longevity were also temperature dependent. Developmental times for each stadium varied from 10 to 24.3 days for stadia one through four at 10°C, 7.7 to 13.5 days for stadia one through five at 26.7°C, and 1.9 to 16.8 days for stadia one through four at 32.2°C. Both male and female longevities were highest at 15.6°C and lowest at 10°C. Number of eggs laid per day per female was (mean+ SD) 605 + 2.5, number of eggs per female per life was (mean + SD) 605 + 190.1. Preoviposition period was 3 to 6 days, and the oviposition period was 11 to 48 days. The optimal temperatures for P. maidis development are 21.1°C and 26.7°C (70).
It was found that several spot characteristics such as the number of tarsomers on the metatarsi, the number of pits on each side of pronotum, the number of teeth on the second tarsomere, and metatibial spurs can be used to identify P. maidis instars (70). P. maidis was reported to utilize such plant hosts as Sorghum bicolor (L.), Pennisetum typhoides (Burm.) Stapf and Hubb, Echinochloa colonum (L.) Link, and Paspalum scrobiculatum L. (56). We have found that this insect was found to breed on S. bicolor, Rottboellia exaltata, Tripsacum dactyloides (73).
III. Corn delphacid transmitted maize mosaic nucleorhabdovirus (MMV):
Maize mosaic was first reported in 1914 in Hawaii (44). It is considered a serious disease in the tropics and subtropics, and has been speculated as a possible cause of the collapse of Mayan civilization (14). The rhabdovirus morphology of maize mosaic virus (MMV) was not known for nearly 40 years (41). MMV is also transmitted by P. maidis in a persistent manner. Maize mosaic has often been confused with maize stripe in the literature because of their similarity. MMV has been reported in Central and South America, Mexico, India, Mauritius, Reunion, Madagascar, and Tanzania (41,75). Although the rhabdovirus infections of maize in the U.S. were found in Texas, Alabama, Louisiana, and Mississippi, positive vector identification and serological relationship to MMV were only done with Florida isolate of MMV (75).
1. Symptomatology and Host Range:

Initial symptoms of maize mosaic are light-green to yellow long stripes along the midrib, these stripes elongate to form the distinct, even, chlorotic stripes between and along the veins extending from the base of the leaf to the tip (Fig. 4).
All commercial maize hybrids tested in Venezuela were susceptible to MMV (46), and all U.S. mainland sweet corn inbreds, hybrids, and cultivars tested in Hawaii were susceptible to MMV (75). Other plants such as R. exaltata, Septaria vulpiseta Roem. E. Schult, S. verticilliflorum Z. mays mexicana Iltis and Sorghum sp. Axonopus compressus Beauv. are susceptible to MMV (6,46,75). Yield losses of more than 50% under glasshouse and field conditions were recorded (6).
2. Properties of MMV:
Various sizes of MMV virions have been reported. Dimensions of 255 X 90 nm for negatively-stained partially-purified preparations, and 242 X 48 nm for particles in thin sections of MMV-infected tissues have been reported for the Venezuelan isolate (46); 224 X 68 nm and 234-325 X 63 nm have been reported for purified virions and those in MMV-infected cells, respectively, for the Florida isolate (10,24); and diminsions of 204 X 67 nm for bullet-shaped particles, 245 X 80 nm for bacilliform particles have been reported for the Hawaiian isolate of MMV (53).
Both the perinuclear accumulation of virus particles in the infected cells (10) and the presence of particle in the cytoplasm of epidermal, mesophyll, and vascular parenchyma cells and phloem and xylem elements of infected plants have been reported (46,53). The granular masses were found to surround the nuclei of the peidermal strips of MMV infected leaves and roots using light microscopy (59).
The virions of MMV have been purified. MMV virions contained a single-stranded RNA of Mr 4.2 X 106 (24). MMV virons contain three major structural proteins of Mr 75,000, 54,000, and 30,000 as analyzed by SDS-PAGE. The Florida isolate of MMV is serologically related to Venezuelan MMV (24), and the three isolates of MMV from Mauritius were also related to Venezuelan MMV (6).
3. Characteristics of MMV Transmission by P. maidis:
MMV is solely transmitted by P. maidis in a persistent-propagative manner. The rate of MMV transmission by P. maidis by means of plant acquisition ranged from 5 to 42% (26,46). P. maidis was able to acquire MMV in less than 15 min. The virus persisted in P. maidis and the patterns of transmission were often erratic (26,75). The median incubation period (IP50) was 13.5 and 14.8 days for Florida isolate of MMV (26). The efficiency of MMV transmission by P. maidis could be increased from 20-43% by injection with either purified MMV or with sap from MMV-infected corn plants (26,46).
The detection of MMV in individuals of P. maidis was dependent on inoculum concentrations (26). The total number of MMV-postive P. maidis decreased with decreasing injection inoculum concentrations (25, 2.5 and 0.25 ug/ml). The average absorbance value for MMV-positive P. maidis increased with time at all three concentrations, indicating multiplication of MMV in P. maidis.
IV. Corn leafhopper transmitted maize rayado fino marafivirus (MRFV):
Maize rayado fino was first reported in El Salvador in the 1960's (5). Later Gamez (30) demonstrated a Costa Rican isolate of maize rayado fino virus (MRFV) transmission by the corn leafhopper, Dalbulus maidis (DeLong and Wolcott). This disease has also been found in Uruguay, Brazil, Colombia, Panama, Guatemala, Honduras, Nicaragua, Mexico, Peru, Venezuela, Ecuador and the U.S. (30,57,66,75). Yield losses in Central America may be up to 40-50% of early infected plants. Losses and incidences may reach 100% for newly introduced cultivars (30). All maize cultivars tested were susceptible to MRFV in Central, South and North America (30,66).
1. Symptomatology and Host Range:

Symptoms on the inoculated maize first appear 7-14 days after inoculation as a few rows of fine, unevenly spaced chlorotic dots or short stripes along the secondary and tertiary veins at the basal portions of the young leaves (Fig. 5).
The discolorations range from chlorosis to complete bleaching. The dots become more numerous and fused longitudinally on succeeding leaves as chlorotic stipple stripes. Symptoms on young plants are always more pronounced than the old plants. However symptoms tend to fade gradually in most inoculated plants. Only Zea mays and its teosinte subspecies, Z. luxurians, Z. diploperennis, Tripsacum australe, Rottboellia exaltata, and several Z. mays X T. dactyloides hybrids were susceptible to MRFV (57).
2. Properties of MRFV:
MRFV can be readily extracted from infected tissue with simple procedures (30). The Texas and Florida isolates of MRFV have been purified by means of chloroform clarification, rate-zonal centrifugation, and isopyncnic banding in CsCl (27,33). MRFV particles are isometric, 22-33 nm in diameter and contain a single-stranded RNA genome (2.4 X 106 daltons) (33). The irregular inclusions and granular inclusions in the parenchyma and phloem cells of leaf sheaths, lef veins and roots were used for diagnosis of MRFV infection (59).
3. Characteristics of MRFV Transmission by D. maidis:
MRFV is transmitted by D. maidis in a persistent manner. A protracted incubation period in the vector is required. The rate of MRFV transmission by D. maidis was usually low ranging from 10-34% (30,57). Nymphs were more efficient transmitters than the adults (57). The average IP in D. maidis varied from 12.5 to 16 days. The average retention period in D. maidis ranged from 16.5-20.2. The infectivity of partially purified MRFV was demonstrated by vector injection and membrane feeding (30). Gonzales and Gamez (35) were first to suggest that MRFV multiplies in D. maidis. Later Nault et al. (57) demonstrated that the transmission rate for D. maidis injected with partially purified MRFV was dosage dependent. Using ELISA tests, MRFV was shown to multiply in D. maidis in a time course study (33,63). The Texas isolate of MRFV has also been experimentally transmitted by D. elimatus, Stirellus bicolor, and Graminella nigrifrons (57). MRFV was reported to be pathogenic to D. maidis and D. elimatus (7).
V. Leafhopper transmitted corn stunt spiroplasma (CSS):
Corn stunt is one of the most economically important diseases of maize in the U.S., Mexico, and Central and South America (55). For many years corn stunt was thought to be caused by several strains of a virus based on symptomatology and vector transmission (37,55). The helical morphology of the causal agent of Rio Grande corn stunt (51) was subsequently established (16,19,77) and was named as corn stunt spiroplasma (CSS) (Spiroplasma kunkelii), CSS is transmitted naturally by D. maidis (DeLong and Wolcott), and& D. elimatus (Ball), and experimentally by Graminella nigrifrons (Forbes), G. sonora (Ball), Stirellus bicolor (Van Duzee), Exitianus exitiosus (Uhler), and Euscelidius variegatus (Kirsch.) (37,55,56).
During 1978-1980, the CSS played a dominant role in the epidemic in south Florida as part of the disease complex (11,72); the infection of CSS in the diseased field samples in 1979 and 1980 reached 68.4 and 98.5%, respectively (11).
1. Symptomatology and Host Range:

The initial symptoms of Rio Grande corn stunt showed characteristic small chlorotic stripes that developed at the leaf bases of new leaves after about 25-30 days. The chlorotic stripes become fused and extended further toward the leaf tips in the older leaves with green spots and stripes on a chlorotic background (Fig. 6). The infected plants had much shorter internodes and a proliferation of secondary shoots in leaf axils.
The reddening on leaves varied depending on the corn genotype and environmental conditions. The plant hosts of CSS are Z. mays, Z. mays mexicana (Schrad.) Iltis, Z. diploperenis Iltis, Doebley and Guzman, Z. perennis (Hitchc.) Reeves and Mangelsd, Z. mays X Tripsacum floridanum Porter ex Vasey L., and Z. luxurians (Durieu and Ascherson) Bird (54,55). In addition, Vicia faba L., Catheranthus roseus (L.) G. Don, and Lolium perenne L. were reported to be susceptible to CSS. Radish (Raphanus sativus), mustard (Sinapis alba) and spinach (Spinacia oleracea) were also reported as experimental hosts for CSS (52).
2. Properties of CSS:
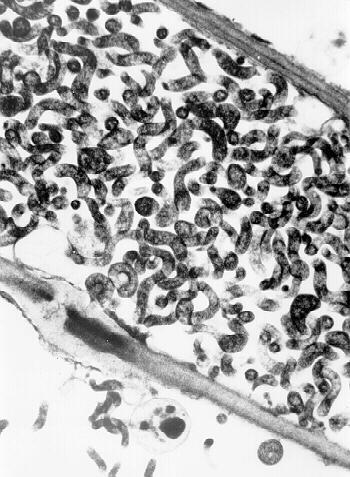
CSS is a motile, helical, cell wall-free prokaryote as seen by phase contrast or dark field microscopy of plant juice or hemolymph and abdominal smears from leafhopper vectors (19). It is a phloem limited organism (Fig. 7).
CSS is highly resistant to penicillin, but sensitive to antibodies in vitro tests, and treatment of inoculated plants with tetracycline antibiotic caused remission of symptoms and interfered with leafhopper transmission (37). CSS was first isolated in an artificial medium, and subsequently was cultured and subcultured (16,20). The successful culture and subculture depended on the method of isolation, incubation temperature, atmospheric conditions, pH, osmotic pressure of the culture medium and composition of medium (75).
CSS was reported to be pathogenic to D. elimatus and D. maidis (39). The pathogenicity of CSS to D. maidis, D. elimatus, D. gelbus, D. guevarai, D. quinquenotatus, D. tripsacoides and Baldulus tripsaci by shortening the longevity of leafhoppers has been demonstrated (48,49,58). Other effects of CSS on leafhopper survival and fecundity (48,49,58), development rates (48) have also been studied.
CSS is not only related to Spiroplasma citri which is the causal agent of citrus stubborn disease, but also to spiroplasmas from honey bees, flower nectar, and ticks. Like other spiroplasmas, it can be infected by spiroplasma virus (20).
3. Characteristics of Leafhopper Transmission:
CSS is transmitted by D. maidis in a persistent and propagative manner (1,56,72). Other vectors include D. elimatus D. grievarai (DeLong), G. nigrifrons, G. sonora, S. bicolor, E. exitosus, Cicadulina mbila (Naude), Macrosteles sexnotatus (Fallen) E. variegatus (52,54,56). D. maidis is the most efficient vector of CSS (2,52) with an AAP of 15 min and 7 days, 15 and 100% of the test insect transmitted CSS respectively, (52). CSS transmission could also be achieved by injection of the vector with either sap extracted from the infected plant or cultured CSS and membrane feeding (16,20,52). 100% transmission efficiency of D. maidis following injection and membrane feeding has been reported (1,2). The IP50 in D. maidis was 19 and 21.2 days (54,55) 17.5 to 21.2 days (72), and 14.3 days (1,2). The length of IP was negatively related to the length of AAP (1,2). The retention periods of CSS by D. maidis were variable ranging from 42 days (1,2), to 45 days (72).
D. guevarai was reported to be a more efficient vector of a Mexican isolate of CSS than D. maidis (62). The rates of CSS transmission by D. elimantus, E. exitiosus, G. nigrifrons, and S. bicolor after a 4-day AAP were at 80, 84, 20, and 61%, respectively (54). The transmission rate of a Jamaican isolate of CSS by C. mbila, E. variegatu and M. sexnotatus were at 60, 3.8 and 2%, respectively (52).
4. Biology of D. maidis

Davis (33) performed a study on the biology of D. maidis at six temperatures and found that D. maidis adult longevity at 70°F was 26-51 days and the average number of eggs produced per female per life was 151, and females failed to lay eggs at 55° and 65°F. Tsai (71) studied the life history of D. maidis at 10, 15.6, 26.7, and 32.2°C. The average development times for instars I-V ranged from 11.6 to 33.6 days at 10°C, 6.3 to 13.3 days at 15.6°C, 2.5 to 3.8 days at 26.7°C, and 2.4 to 4.4 days at 32.2°C. Both male and female (Fig. 8) longevities were greatest at 15.6°C and lowest at 32.2°C.
Oviposition data obtained at 15.6 and 26.7°C, respectively, showed that the number (x + SD) of eggs per female per day was 3.62 + 1.09 at 15.6°C and 14.18 + 3.55 at 26.7°C, the number (x + SD) of eggs per female per life was 402.33 + 140.03 at 15.6oC and 611.08 + 164.96 at 26.7°C. Eggs were seldom laid within 24 hours after adult emergence. Adult longevities (x + SD) between mated females and unmated females at 15.6 and 26.7°C were 111.00 + 14.54 days for mated, and 180.00 + 26.09 days for unmated at 15.6oC, and 45.15 + 15.81 days for mated and 112.00 + 16.52 days for unmated at 26.7°C. Pitre (60) reported that the nymphal development time on corn ranged from 11 to 16 days and the average adult longevity was 12.2 and 12.1 days for females and males, respectively. Besides corn, teosinte, Euchlaena mexicana and gamma grass, Tripsacum dactyloides were also reported as alternate hosts of D. maidis (61). The mean nymphal development time on T. dactyloides was 15.4 and 15.3 days and the average adult longevity was 33 and 11.6 days for females and males, respectively (60). Tsai (71) also tested T. dactyloides, T. dactyloides var. meridonale, T. floridanum, Rottboellia exaltata, Secale cereale, and Avena sativa as alternate hosts for D. maidis and found that only T. dactyloides var. meridonale was suitable for rearing D. maidis.
VI. Leafhopper transmitted maize bushy stunt phytoplasma (MBSM):
In 1955, Maramorosch described two types of corn stunt from Mexico. The first type was designated as Rio Grande corn stunt originally described Kunkel (45) from Texas. The second type was designated as Mesa Central corn stunt which is now believed to be a nonhelical mycoplasma, the maize bushy stunt mycoplasma (MBSM) (54). Maramorosch (51) futher demonstrated the difference of these two agents by means of cross protection between maize bushy mycoplasma and CSS. Based on symptomatology, the Louisiana and Mississippi corn stunt agents could also be MBSM (36,38,75). However the proof of their identity and relationship awaits culture of the MBSM and serological study.
1. Symptomatology and Host Range:
Inoculated plants initially develop a marginal yellow and orange color of the older leaves. The symptoms on subsequently developed leaves are characterized by marginal chlorosis, tearing, shortening and twisting of young leaf tips. Numerous tillers develop at the base of the plant and at leaf axils. Sweet corn cultivars such as "Aristogold" and "Aritogold Bantam Evergreen" hybrids develop extensive leaf reddening, and more basal and auxillary shoots as compared to "Guardian" sweet corn (Fig. 9). Only maize and three races of Z. mays L. mexicana

2. Biology of MBSM:
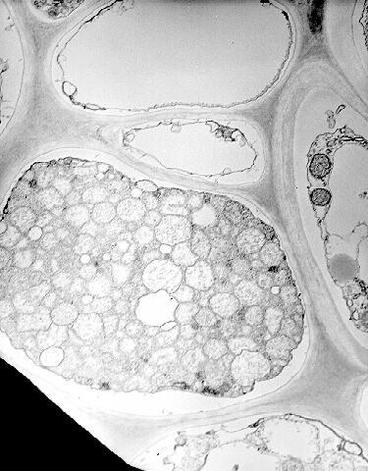
MBSM is a phloem limited phytoplasma and is similar in morphology and ultrastructure to that of yellows diseases of plants (Fig. 10). Granados (36) demonstrated the occurrence of Louisiana corn stunt agent both in vectors and plants. MBSM has not been cultured in vitro. Recently, cloned DNA probes for detecting MBSM from the experimentally inoculated corn and infectious D. maidis were developed (22).
3. Transmission Characteristics of MBSM by Leafhopper Vectors:
MBSM is transmitted by D. maidis in a peristent and propagative manner. It is also transmitted by D. elimatus, Baldulus tripsaci, G. nigrifrons, and G. sonorus (36,38,54,55). The rate of MBSM transmission by D. maidis ranged from 43.9 to 88.9% (54,72) whereas it was transmitted by D. elimatus and G. nigrifrons at 58.8 and 8.2%, respectively (54). The mean IP for Texas isolate of MBSM in D. maidis was 24.2 and 25.5 days (54,72) and IP50 for Florida islate of MBSM in D. maidis was 28 days (72). The minimum IP for the Louisiana isolate of corn stunt was 17 to 22 days for D. maidis, 14 to 15 days for D. elimatus and 22 to 26 days for G. nigrifrons (38). The minimum IP for Mississippi isolate was 12 days for D. maidis and 15 to 18 days for G. nigrifrons. The average retention time for Texas and Florida isolates of MBSM was 32.6 and 34.5 days, respectively (72). Under field conditions, plants were often found to be doubly- infected with CSS and MBSM (55,75). However, the symptoms of MBSM in the doubly- infected plants always developed earlier than those of CSS as the incubation period for MBSM in corn was 18.9 days compared to 43.3 days for CSS (55).
The Prospect of control:
Disease and/or pest control is the ultimate goal of each researcher. The complexity of insect-transmitted corn pathogens makes them more difficult to control. The most widely employed practice at present time is integrated pest management (3). However, the most promising control measure is the use of resistant or tolerant varieties. To date the only successful example in developing the major breeding program for both dent and sweet corn resistant to MMV is done by Brewbaker (13). No corn lines immune to MStV, MRFV, CSS, MBSM are available. This poses a tremendous challenge for maize genetisists and breeders to identify the gene(s) responsible for the resistance in maize germplasm. Any fruitful results will probably have to come from the concerted effort of molecular biologists, plant breeders, plant pathologists and vector entomologists.
We are all aware of the recent advances in biotechnology. Its promise is so powerful that it cuts across disciplines of biological sciences and offers us a promising tool to address the complex problems of maize. By using the techniques of protoplast fusion, in vitro plant culture and recombinant DNA technology, one should be able to hybridize corn with the perennial relatives of maize among Zea and Tripsacum spp. which are tolerant or immune to CSS and MBSM, or perhaps with other sexually incompatible monocotyledon species as well as to develop better control of the pests. Biotechnology potentially cuts down the time required for corn variety breeding and propagation, and it provides maize researchers with a good method for protecting and exchanging germplasm.
References
- Alivizatos, A. S., and Markham, P. G. 1986. Multiplication of corn stunt spiroplasma in Dalbulus maidis and transmission in vitro, following injection. Ann. Appl. Biol. 108:545-554 Alivizatos, A. S., and Markham, P. G. 1986.
- Acquisition and transmission of corn stunt spiroplasma by its leafhopper vector Dalbulus maidis. Ann. Appl. Biol. 108:535-544
- All, J. N. 1983. Integrating techniques of vector and weed-host suppression into control programs for maize virus diseases. pp. 243-247 In: Proc. Int'l. Maize Virus Dis. Colloq. and Workshop, 2-6 August 1982. D.T. Gordon, J. K. Knoke, L. R. Nault, and R. M. Ritter, (eds.). The Ohio State Univ., Ohio Research and Development Center, Wooster. 266 pp.
- Ammar, E. D., Gingery, R. E., and Nault, L. R. 1985. Two types of inclusions in maize infected with maize stripe virus. Phytopathology 75:84-89
- Ancalmo, O., and Davis, W. C. 1961. Achaparramiento (corn stunt). Plant Dis. Rep. 45:281. 6. Autrey, L. J. C. 1983. Maize mosaic virus and other maize virus diseases in the islands of the Western Indian Ocean. pp. 167-181 In: Proc. Int'l. Maize Virus Dis. Colloq. and Workshop. 2-6 August 1982. D. T. Gordon, J. K. Knoke, L. R. Nault, and R. M. Ritter (eds.). The Ohio State University, Ohio Agricultural Research and Development Center, Wooster. 266 pp.
- Bacardo, L. E., Graziano, J. V., Montessaro, R. R., and Majica, H. B. 1984. Tablas de vida y fertilidad de poblaciones de Dalbulus maidis DeLong & Wolcott y Dalbulus elimatus Ball (Homoptera: Cicadellidae) transmisoras y no transmisoras del virus del rayado fino del maiz. Agrociencia 57: 195-205
- Bock, K. R., Guthrie, E. J., and Woods, R. D. 1974. Purification of maize streak virus and its relation-ship to viruses associated with streak diseases of sugarcane and Panicum maximum. Ann. Appl. Biol. 77:289-296
- Boothroyd, C. W. 1977. Seed transmission of maize dwarf mosaic virus in sweet corn and yield reduction in plants from an infected seed lot (Abstract) Proc. Am. Phytopathol. Soc. 4:184.
- Bradfute, O. E., and Tsai, J. H. 1983. Identification of maize mosaic virus in Florida. Plant Dis. 67:1339-1342
- Bradfute, O. E., Tsai, J. H., and Gordon, D. T. 1981. Corn stunt spirolplasma and viruses associated with a maize disease epidemic in southern Florida. Plant Dis. 65:837-841.
- Bradfute, O. E., and Tsai, J. H. 1990. Rapid identification of maize stripe virus. Phytopathology 80:715-719.
- Brewbaker, J. L. 1975. Resistance to maize mosaic virus I in Hawaii. pp. 4-5 In: Corn and Sorghum Diseases and Insect Pests in Hawaii. Hawaii Agric. Exp. Stn. Misc. Publ. 122, Univ. Hawaii. 22 pp.
- Brewbaker, J. L. 1980. Diseases of maize in the wet lowland tropics and the collapse of the Maya civilization. Econ. Bot. 33:101-118.
- Chen, C. C., Tsai, J. H., Chiu, R. J., and Chen, M. J. 1993. Purification, characterizaiton, and serological analysis of maize stripe virus in Taiwan. Plant Dis. 77:367-372. 16. Chen, T. A., and Liao, C. H. 1975. Corn stunt spiroplasma: isolation, cultivation, and proof of pathogenicity. Science 188:1015-1017.
- Chen, C. C., Chao, C. H. Chen, Y. K. and Tsai, J. H. 1996. Comparative studies on the partial properties of three tenuiviruses occurring in Taiwan. Taichung District Agric. Improv. Sta. Research Bull. 50:29-43
- Davis, R. 1966. Biology of the leafhopper Dalbulus maidis at selected temperatures. J. Econ. Entomol. 59:766.
- Davis, R. E., and Worley, J. F. 1973. Spiroplasma: Motile, helical microorganism associated with corn stunt disease. Phytopathology 63:403-408
- Davis, R. E., Chen, T. A., and Worley, J. F. 1981. Corn stunt spiroplasma. pp. 40-50 In: Virus and Viruslike Diseases of Maize in the United States. D. T. Gordon, J. K. Knoke, and G. E. Scott (eds.). South. Coop. Ser. Bull. 247. 218 pp.
- Davis, M. J., Tsai, J. H., Cox, R. L., McDaniel, L. L., and Harrison, N.A. 1988. Cloning of chromosomal and extrachromosomal DNA of the mycoplasmalike organism that causes maize bushy stunt disease. Molecular Plant-Microbe Interactions. 1:295-302.
- Estabrook, E. M., Suyenaga, K., Tsai, J. H. and Falk, B. W. 1996. Maize stripe tenuivirus RNA2 is ambisense and encodes a protein similar to the Phlebovirus virion membrane glycoproteins. Virus Gene (In press)
- Falk, B. W., and Tsai, J. H. 1983. Assay for maize stripe virus-infected plants by antiserum produced to a purified noncapsid protein. Phytopathology 73:1259-1262.
- Falk, B. W., and Tsai, J. H. 1983. Physicochemical characterization of maize mosaic virus. Phytopathology 73:1536-1539.
- Falk, B. W., and Tsai, J. H. 1984. Identification of single- and double-stranded RNAs associated with maize stripe virus. Phytopathology 74:909-915
- Falk, B. W., and Tsai, J. H. 1985. Serological detection and evidence for multiplication of maize mosaic virus in the planthopper, Peregrinus maidis. Phytopathology 75:852-855.
- Falk, B. W., and Tsai, J. H. 1986. The two capsid proteins of maize rayado fino virus contain common peptide sequences. Intervirology 25:111-116.
- Falk, B. W., Tsai, J. H., and Lommell, S. A. 1987. Differences in levels of detection for maize stripe virus capsid and major noncapsid proteins in plant and insect hosts. J. Gen. Virol. 68:1801-1811.
- Huiet, L., Tsai, J. H. and Falk, B. W. 1993. Maize stripe virus RNA5 is of negative polarity and encodes a highly basic protein. J. Gen. Virol. 74:549-554
- Gamez, R. 1983. Maize rayado fino disease: The virus-host-vector interaction in neotropical environments. pp. 62-68. In: Proc. Int'l. Maize Virus Dis. Colloq. and Workshop, 2-6 August 1982. D. T. Gordon, J. K. Knoke, L. R. Nault, and R. M. Ritter (eds.). The Ohio State University, Ohio Agricultural Research and Development Center, Wooster. 266 pp.
- Gingery, R. 1988. The rice stripe virus group. pp. 297-329. In: The Plant Viruses 4: The Filamentous Plant Viruses. R. G. Milne (ed.). Plenum, New York
- Gingery, R. E., Nault, L. R., and Bradfute, O. E. 1981. Maize stripe virus: Characteristics of a member of a new virus class. Virology 112:99-108.
- Gingery, R. E., Gordon, D. T., and Nault, L. R. 1982. Purification and properties of an isolate of maize rayado fino virus from the United States. Phytopathology 72:1313-1318.
- Gingery, R. E., Nault, L. R., and Yamashita, S. 1983. Relationship between maize stripe virus and rice stripe virus. J. Gen. Virol. 64:1765-1770.
- Gonzalez, V., and Gamez, R. 1974. Algunos factores que afectan la transmision del virus del rayado fino del maiz por Dalbulus maidis (DeLong and Wolcott). Turrialba 24:51-57.
- Granados, R. R. 1969. Maize viruses and vectors. pp. 327-359 In: Viruses, Vectors, and Vegetation. K. Maramorosch, (ed.). Interscience Publishers, New York, NY. 666 pp
- Granados, R. R. 1969. Chemotherapy of the corn stunt disease (Abstr.) Phytopathology 59:1556
- Granados, R. R., Granados, J. S., Maramorosch, K., and Reinitz, J. 1968. Corn stunt virus: Transmission by three cicadellid vectors. J. Econ. Entomol. 61:1282-1287
- Granados, R. R., and Meehan, D. J. 1975. Pathogenicity of the corn stunt agent to an insect vector, Dalbulus maidis. J. Invetebr. Pathol. 26:313-320.
- Greber, R. S. 1983. Characteristics of viruses affecting maize in Australia. pp. 206-218. In: Proc. Int'l. Maize Virus Dis. Colloq. and Workshop. 2-6 August 1982. D. T. Gordon, J. K. Knoke, L. R. Nault, and R. M. Ritter (eds.). The Ohio State University, Ohio Agricultural Research and Development Center, Wooster. 266 pp.
- Herold, F. 1972. Maize mosaic virus. No. 94 In: Descriptions of Plant Viruses. Commonw. Mycol. Inst., Assoc. Appl. Biologists, Kew, Surrey, England. 4 pp.
- Knoke, J. K., Anderson, R. J., Louie, R., Modelen, L. V., and Findley, W. R. 1983. Insect vectors of maize dwarf mosaic virus and maize chlorotic dwarf virus. pp. 130-138. In: Gordon, D. T., Knoke, J. K., Nault, L. R., and Ritter, R. M. eds. Proc. Int'l. Maize Virus Disease Calloq. and Workshop. 1982. Ohio Agric. Res. and Dev. Center, Wooster
- Kulkarni, H. Y. 1973. Comparison and characterization of maize stripe and maize line viruses. Ann. Appl. Biol. 75:205-216.
- Kunkel, L. O. 1921. A possible causative agent for the mosaic disease of corn. Hawaii. Sugar Plant. Assoc. Exp. Stn. Bull. Bot. Ser. 3:44-58.
- Kunkel, L. O. 1948. Studies on a new corn virus disease. Arch. Gesamte Virusforsch. 4:24-46
- Lastra, R. J. 1977. Maize mosaic and other maize virus and virus-like diseases in Venezuela. pp. 30-39 In: Proc. Int'l. Maize Virus Dis. Colloq. and Workshop, 16-19 Aug., 1976. L. E. Williams, D. T. Gordon, and L. R. Nault, (eds.). Ohio Agric. Res. Dev. Cent., Wooster. 145 pp
- MacKenzie, D. R. Wernham, C. C. and Ford, R. E. 1966. Differences in maize dwarf mosaic virus isolates of the northeatern United States. Plant Dis. Rep. 50:814-818 48. Madden, L. V., and Nault, L. R. 1983. Differential pathogenicity of corn stunting mollicutes to leafhopper vectors in Dalbulus and Baldulus species. Phytopathology 73:1608-1614.
- Madden, L. V., Nault, L. R., Heady, S. E., and Styer, W. E. 1985. Effect of maize stunting mollicutes on survival and fecundity of Dalbulus leafhopper vectors. Ann. Appl. Biol. 105:431-441.
- Maramorosch, K. 1955. The occurrence of two distinct types of corn stunt in Mexico. Plant Dis. Rep. 39:896-898.
- Maramorosch, K. 1958. Cross protection between two strains of corn stunt virus in an insect vector. Virology 6:448-459.
- Markham, P. G., and Alivizatos, A. S. 1983. The transmission of corn stunt spiroplasma by natural and experimental vectors. pp. 56-61 In: Proc. Int'l. Maize Virus Dis. Colloq. and Workshop. 2-6 August 1982. D. T. Gordon, J. K. Knoke, L. R. Nault, and R. M. Ritter (eds.). The Ohio State University, Ohio Agricultural Res. and Dev. Cent., Wooster. 266 pp.
- McDaniel, L. L., Ammar, E.-D., and Gordon, D. T. 1985. Assembly, morphology, and accumulation of a Hawaiian isolate of maize mosaic virus in maize. Phytopathology 75:1167-1172.
- Nault, L. R. 1980. Maize bushy stunt and corn stunt: A comparison of disease symptoms, pathogen host ranges, and vectors. Phytopathology 70:659-662.
- Nault, L. R., and Bradfute, O. E. 1979. Corn stunt: Involvement of a complex of leafhopper-borne pathogens. pp. 561-586 In: Leafhopper Vectors and Plant Disease Agents. K. Maramorosch and K. F. Harris (eds.). Academic Press, New York, NY. 654 pp.
- Nault, L. R., and Knoke, J. K. 1981. Maize vectors. pp. 77-84 In: Virus and Viruslike Diseases of Maize in the United States. D. T. Gordon, J. K. Knoke, and G. E. Scott, (eds.). Southern Cooperative Series Bull. 247. June 1981. 218 pp
- Nault, L. R., Gingery, R. E., and Gordon, D. T. 1980. Leafhopper transmission and host range of maize rayado fino virus. Phytopathology 70:709-712
- Nault,, L. R., Madden, L. V., Styer, W. E., Triplehorn, B. W., Shambaugh, G. F., and Heady, S. E. 1984. Pathogenicity of corn stunt spiroplasma and maize bushy stunt mycoplasma to its vector Dalbulus longulus. Phytopathology 74:977-979.
- Overman, M. A., Ko, N. J., and Tsai, J. H. 1992. Identification of viruses and mycoplasmas in maize by light microscopy. Plant Dis. 76:318-322.
- Pitre, H. N. 1970. Observations on the life cycle of Dalbulus maidis on three plant species. Fla. Entomol. 53:33-3
- Pitre, H. N., Combs, R. L., and Douglas, W. A. 1966. Gamagrass, Tripsacum dactyloides: A new hostof Dalbulus maidis, vector of corn stunt virus. Plant Dis. Rep. 50:570-571.
- Ramirez, J. L., DeLeon, C., Garcia, C., and Granados, G. 1975. Dalbulus guevarai (DeL.) nuevo vector del achaparramiento del maiz en Mexico: Incidencia de la enfermedad y su relacion ocn el vector Dalbulus maidis (DeL. & W.) en Muna. Yucatan. Agrociencia 22:39-49
- Rivera, C., and Gamez, R. 1986. Mustiplication of maize rayado fino virus in the leafhopper vector Dalbulus maidis. Intervirology 25: 76-83.
- Rosenkranz, E. 1981. Host range of maize dwarf mosaic virus. pp. 152-162. In: Gordon, D. T., Knoke, J. K. and Scott, G. E. eds. Virus and viruslike diseases of maize in the United States. Southern Coop. Series Bulletin 247. June 1981. 218 pp.
- Storey, H. H. 1936. Virus diseases of East African plants. IV. A Survey of the viruses attacking the Gramineae. East Afr. Agric. J. 1:333-337.
- Toler, R. W., Skinner, G., Bockholt, A. J., and Harris, K. F. 1985. Reactions of maize (Zea mays) accessions to maize rayado fino virus. Plant Dis. 68:56-57.
- Toriyama, S. 1982. Characterization of rice stripe virus: A heavy component carrying infectivity. J. Gen. Virol. 61: 187-195.
- Tsai, J. H. 1975. Occurrence of a corn disease in Florida transmitted by Peregrinus maidis. Plant Dis. Rep. 59:830-833.
- Tsai, J. H., and Zitter, T. A. 1982. Transmission characteristics of maize stripe virus by the corn Delphacid. J. Econ. Entomol. 75:397-411.
- Tsai, J. H., and Wilson, S. W. 1986. Biology of Peregrinus maidis with descriptions of immature stages (Homoptera: Delphacidae). Ann. Entomol. Soc. Am. 79:395-401
- Tsai, J. H. 1987. Bionomics of Dalbulus maidis (DeLong and Wolcott). A vector of mollicutes and virus (Homoptera: Cicadellidae). In: Mycoplasma Diseases of Crops: Basic and Applied Aspects. K. Maramorosch and S. P. Raychaudhuri (eds.). Springer Verlag. New York, NY.
- Tsai, J. H. 1987. Mycoplasma diseases of corn in Florida. In: Mycoplasma Diseases of Crops: Basic and Applied Aspects. K. Maramorosch and S. P. Raychaudhuri (eds.). Springer Verlag. New York, NY.
- Tsai, J. H. 1996. Development and oviposition of Peregrinus maidis (Homoptera: Delphacidae) on various host plants. Fla. Entomol. 70:19-26
- Tsai, J. H. and Brown, L. G. 1989. Maize dwarf mosaic virus. Division of Plant Industry. Fla. Dept. Agric. & Consumer Serv. Plant Path. Circular. No. 320
- Tsai, J. H. and Falk, B. W. 1993. Viruses and mycoplasmal agents affecting maize in the Tropics. pp. 43-48. In: Proc. Symposium on Plant Virus and Virus-like Diseases. R. J. Chiu and Y. Yeh (eds.) 434. pp
- Williams, L. E. and Alexander, L. J. 1965. Maize dwarf mosaic, a new corn disease. Phytopathology 55:802-804
- Williamson, D. L., and Whitcomb, R. F. 1975. Plant mycoplasma: A cultivable spiroplasma causes corn stunt disease. Science 188:1018-1020.