Alan C. Bartlett
USDA, ARS,
Western Cotton Research Laboratory
Robert T Staten
USDA, APHIS, PPQ,
Phoenix Methods Development Center,
Phoenix, AZ 85040
Induced Sterility in Insects
History

Discoveries in such diverse disciplines of science as radiation biology, chemistry, animal behavior, molecular biology, genetics and entomology have contributed to our current ideas concerning the control of insect pests. In 1895 W. K. Röntgen observed and reported his discovery of X-rays. By 1903 the effects of such rays from radium upon the reproductive systems and development of insects had been reported by C. Bohn. G. A. Runner, in 1916, found that low doses of X-rays decreased the reproductive capacity of the cigarette beetle, while higher doses killed them.
By 1926 H.J. Muller had demonstrated that X-ray radiation will cause heritable changes in fruit flies (Drosophila melanogaster Meigen). Dominant lethal mutations were found to be one of the most frequent types of induced mutation. The "partial sterility" of X-ray treated males was of concern to Muller because this effect reduced the numbers of visible mutations which could be recovered for a given dose of radiation.
As early as 1937, E. F. Knipling had conceived an approach to insect control in which the natural reproductive processes of the screwworm fly (Cochliomyia hominivorax Coquerel) are disrupted by chemical or physical mechanisms thus rendering the insects sterile (Knipling 1985). These sterile insects are then released into the environment in very large numbers (10 to 100 times the number of native insects) in order to mate with the native insects that are present in the environment. A native female that mates with a sterile male will produce eggs, but the eggs will not hatch (the same effect will occur for the reciprocal cross). Since there are 10 to 100 more sterile insects in the population than native insects, most of the crosses are sterile. As time goes on, the number of native insects decreases and the ratio of sterile to normal insects increases, thus driving the native population to extinction.
This unique insect control method is now known as the sterile insect technique (SIT), or the sterile insect release method (SIRM). SIT proved to be an extremely successful method for control of the screwworm fly when ionizing radiation was used as the sterilizing source. The success of the screwworm SIT program led to investigations of the effects of radiation on the reproductive performance of many other economically important insects.
The use of mutagenic chemicals to sterilize insects for sterile insect release programs was also considered because this seemed to be an efficient method of causing sterility (Knipling 1979). For example, chemical sterilants could be added to the rearing diet, or the chemical could be applied to eggs or pupae at times when they are being handled in the normal rearing process. However, the use of chemical sterilants is presently very limited because of fears of environmental contamination by insects carrying carcinogenic materials. There are also problems with disposing of the diet or other carriers used to administer the chemosterilant to the insects. Certain types of photo-chemically reactive dyes (e. g. D&C red number 28) have been demonstrated to have insecticidal properties but are environmentally safe. There is a challenge for investigators to discover similar chemicals that could be used as sterilants.
The methods that are presently employed for the successful use of the sterility principle (Bartlett 1990) have not changed significantly since E. F. Knipling's original formulation:
- Techniques that make it possible to produce large numbers of the target insect. (Rearing component)
- Techniques that make it possible to sterilize large numbers of the target insect. (Treatment component)
- Reasonably competitive insects that can be released after sterilization. (Competitiveness component)
- Economical systems for releasing and distributing the insects over the treatment area. (Release component)
- Tools that will assess native populations accurately before and after the release of the treated insects. (Evaluation component)
- A treatment area large enough (or well-isolated enough) to exclude the possibility of immigration of inseminated females into the release area. (Re-infestation component)
There are difficulties encountered when SIT is applied to certain Lepidoptera, Coleoptera, or to insects (like mosquitoes and houseflies) whose obnoxious behavior precludes the release of large numbers in the vicinity of people or animals. For example, a dose of 5500 rad (55 Gy) is sufficient to guarantee 100% sterility of screwworm flies. However, even after doses exceeding 50,000 rad (500 Gy), some lepidopteran pests will still produce F1 progeny. Investigators have found that these F1 progeny exhibit levels of sterility equal to or higher than those of their treated parents. This inherited (or delayed) sterility has been suggested to be of use in control programs (Anonymous 1993).
The existence of insect species that do not respond to our attempts at sterilization has led investigators to search for other methods which use genetic principles, but do not necessarily involve radiation-induced sterility. These other "autocidal" control methods are discussed in more detail after we discuss the cases where induced sterility has been used.
Insect Control Programs Using Induces Sterility
The successful use of sterile insects for the eradication of the screwworm from the United States and Mexico has been detailed in a number of symposia and books. The USDA Animal and Plant Health Inspection Service maintains a home page on the screwworm eradication program. The screwworm program has had its share of skeptics and detractors who theorized that the sterile insect release method could never work (see a discussion of some pro's and con's concerning insect eradication in Cox 1978). In spite of all the scientific criticism, eradication proceeded quickly across the southern tier of the United States. The US portion of the screwworm eradication program started in Florida in 1957 and by 1966 all self-sustaining screwworm colonies in the US were eliminated. However re-infestations from flies migrating from Mexico compromised the program, so in 1972 a joint United States-Mexico program was initiated. This program enabled Mexico to be officially declared free of screwworms in 1991, Belize and Guatemala in 1994, and El Salvador in 1995. Honduras is considered technically free of screwworms since no flies have been detected since January, 1995. The ultimate goal of a proposed United States-Central America project is to maintain a sterile insect barrier at the Darien Gap in Panama starting in 1997. This program has saved billions of dollars in livestock and wildlife loses since its inception.
Although the screwworm eradication program is the most visible and successful use of SIT, a number of other insect species have been subjected to the release of sterile insects with varying success. This includes the following pests and areas where programs have been carried out (this is not an all-inclusive list): (1) screwworm fly /USA, Mexico, Libya; (2) Mediterranean Fruit Fly (Ceratitis capitata Wiedemann)/USA (California), Mexico; (3) Melon Fly (Dacus cucurbitae Coquillett)/Japan, Taiwan; (4) Pink Bollworm (Pectinophora gossypiella Saunders)/USA (California); (5) Tsetse Fly (Glossina species)/Tanzania, Zimbabwe, Upper Volta; (6) Mosquitoes (various) USA (Florida), East Africa, Venezuela; (7) Boll Weevil (Anthonomus grandis Boheman)/Southeastern USA; (8) Mexican Fruit Fly, (Anastrepha ludens Loew)/USA (Texas), Mexico; (9) Gypsy Moth (Lymantria dispar Linnaeus)/Northeastern USA, Canada; (10) Stable Fly (Stomoxys calcitrans Linnaeus)/USA (St. Croix, Virgin Islands - experimental); (11) Horn Fly (Haematobia irritans Linnaeus)/USA (Texas - experimental); (12) Corn Earworm (Helicoverpa zea Boddie)/USA (St. Croix, VI); (13) Tobacco Hornworm (Manduca sexta Linnaeus)/USA (St. Croix, VI).

One of the more successful and long-term sterile insect release programs involves the pink bollworm in the San Joaquin Valley of California (Staten et al. 1993). Over a 24 year period there has been a continuous release of sterile pink bollworm adults during each day of the cotton growing season. This program was initiated in 1968 to protect the ca 500,000 ha of cotton in the valley from infestation by the pink bollworm. In other words, this program was conceived as a barrier against the high populations of pink bollworms found in Arizona, Southern California, and Northern Mexico during the decades of 1950 and 1960.
The pink bollworm program has been a cooperative effort of the United States Department of Agriculture, the California Department of Food and Agriculture and the California Cotton Pest Control Board. From 1970 to 1991 the pink bollworm rearing facility in Phoenix produced from 99 million to 826 million dye-marked moths per year. Most of the moths were radiation sterilized and released from airplanes over cotton natives in the San Joaquin; however, large numbers of moths have also been provided for research programs on sterility, insecticide evaluation, and pheromone isolation and identification.

During the course of this release program, the numbers of native moths captured in the San Joaquin Valley has varied considerably from year to year (Staten et al. 1993). Because of careful monitoring of the dye-marked sterile and native populations through the use of pheromone baited traps, the program was able to quickly adjust both the patterns of release and numbers of sterile insects released in a given area. Each year releases are made in sections of cotton which had native moth captures the previous year. As native pink bollworms appear in trap captures, the release patterns and rates are adjusted accordingly. In only one year (1990), out of 24 years of the program, have conventional insecticides been used to control pink bollworm infestations and then on a very limited basis (two sprays on 280 acres out of 1184 total). No carry-over of this population from 1990 to 1991 was apparent, and overall numbers of native captures in 1992 were low. To the present time, no other detectable economically damaging populations of pink bollworm have developed in the San Joaquin Valley.
Other Autocidal Insect Control Techniques
Some species of insects have not proved to be good candidates for the sterile insect technique, so other methods of using the insect to control itself (autocidal control) have been investigated. The following approaches have been investigated (see Davidson 1974, Pal and Whitten 1974, and Whitten 1985, for some early discussion of the use of genetics in insect control).
Inherited Sterility
This effect has also been referred to as: delayed sterility, F1 sterility, partial sterility, etc. This phenomenon is of use in organisms, such as Lepidoptera and Homoptera, that contain polycentric chromosomes. It involves the transmission of aberrant chromosomes (usually in the form of translocations) from the released population to the native population.
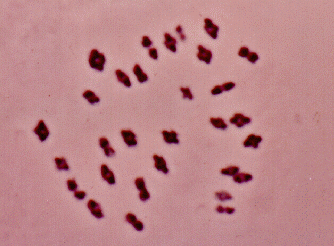
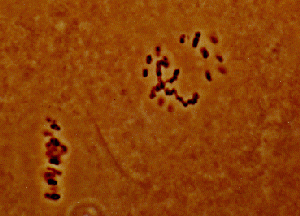
This can be accomplished in two ways: 1) by treating a laboratory-reared population of insects with a dose of radiation which is sufficient to break chromosomes, but low enough to produce little direct sterility, or 2) by culturing stocks of the target species which contains homozygous translocations (this is rather difficult and tedious to accomplish). When the aberrant chromosomes are passed on to the native populations, the individuals carrying the aberrations in the heterozygous state will show sterility. The amount of sterility will depend on the number and size of the translocations carried by each individual. Some advantages of this method include: 1) enhanced reproductive competitiveness of the partially sterile released individuals compared to the fully sterilized individuals used in SIRM; 2) the F1 individuals are produced in the native population, thus freeing rearing facilities for the production of higher numbers of the primary release organism (Anonymous 1993).
Conditional Lethal Mutations
In this technique, strains of insect are produced (by genetic manipulation) carrying traits that are detrimental to the species in the native environment, but which are not detrimental under laboratory conditions. For example, the inability to diapause would be a conditional lethal in an insect that had to go into diapause to survive a host-free period, but in the laboratory no diapause would be necessary. Some other types of conditional lethal traits which could be used are cold-sensitive lethal mutations, high-temperature-sensitive lethal mutations, inability to fly, lack of sex pheromone production or response, lack of ability to develop on a particular host, change in protective coloration, or any other genetic change which could affect an individual's ability to survive or reproduce in the native. In the past, the introduction of conditional lethals was dependent upon our ability to identify and isolate induced or naturally occurring mutations in the target species. Investigators are presently investigating the possibility that such mutations can be taken from genetically well known organisms, such as D. melanogaster, and introduced into economically important organisms using new techniques developed in molecular biology (Cockburn, et al. 1989)
Behavioral Changes
It would be advantageous to cause changes in the specific insect behaviors that make them undesirable or destructive. For example, in many pest species, a change from multi-voltine to univoltine development would eliminate the economic destructiveness of the pest. Such a change is often shown to be genetic in character. If we could learn enough about insect physiology to be able to identify the genetic determinants behind such behavior, then we should be able to genetically manipulate the species and release the new strain in such large numbers that the new trait would become predominant in the population. Some other such traits would be alterations of host range, time of development, fecundity, sex-ratio, humidity requirements, pupation sites, etc. We should also consider traits in insects that would make the insect susceptible to cheaper or environmentally benign chemicals.
Hybrid Sterility
The evolutionary background of many insect pests leads us to believe that reproductive barriers may exist between races of the same species in different geographic areas or between closely related species. Sterile hybrids could be raised in the laboratory and released into native populations. This phenomenon has been clearly demonstrated in crosses between Heliothis virescens males and Heliothis subflexa females (Laster et al. 1996) and should be more widely investigated.
Simply Inherited Mutations
Genetic changes in a single gene could lead to decreased fitness in the native if the gene could be forced into the native population in large numbers. Some examples of such changes would be recessive lethal mutations, eye color changes (to increase or decrease light sensitivity), pupal or adult body color changes, antennae, leg, or wing deformities.
It is obvious that each of the previously mentioned phenomena requires significant research data on insect genetics, physiology, behavior, and reproduction to bring any of the techniques to fruition.
Genetic Sexing Techniques
In many applications of autocidal control, it would be efficient to separate the males and females before release. Possible reasons for such separation are to avoid assortative mating; to avoid any increase in the size of the feral population during a genetic control procedure; to eliminate females which may be disease vectors, extremely obnoxious, or which cause damage to produce or livestock. Another reason for using a genetic sexing procedure is to bring about cost savings in the rearing process if one sex could be eliminated in the egg stage (pre-zygotic sexing). In this case, twice as many insects (of one sex) could be produced with a given expenditure for diet and labor.
Thus, removal of females during the rearing process has been a goal in autocidal control research for many pest insects. The most successful efforts have been with a mosquito, Anopheles albimanus, the medfly, Ceratitis capitata, and the stable fly, Stomoxys calcitrans. Two of these approaches demonstrate what can be done. A combination of mechanical separation of the sexes and genetic manipulation has been employed in the medfly. Wild-type pupal color is brown. Mutants involving either black pupae or white pupae have been linked with the sex chromosomes by means of single or multiple chromosomal translocations. By this means strains are produced in which male pupae are black and female pupae are brown. The pupae are then separated by electronic color recognition circuitry in a mechanical or air-driven sorter. Some disadvantages of this technique are the possible breakdown of the translocation stocks due to crossing-over, contamination of the strain by wild-type individuals, and partial sterility of the strain due to the translocation.
If no use can be found for the reared females, then it would be worthwhile to eliminate them before they consume expensive diet. A number of schemes have been proposed to accomplish removal of females at the egg or early larval stage. In some species, alleles resistant to specific toxic chemicals, such as ethyl alcohol, endrin, purine, potassium sorbate, dieldrin, cyromazine, and propoxur have been selected. Then translocations between one of the sex-chromosomes (usually the Y-chromosome) and the chromosome bearing the resistance locus are induced. When the toxic substance is introduced into the colony, only the sex carrying the resistant allele survives. This method has been successful for three species of mosquitoes and is being actively investigated for the medfly.
Molecular Genetic Techniques
The demonstration of genetic transformation of D. melanogaster with transposable vectors (P-elements) raised the hope that similar techniques could be employed in other insect species. At the least, the demonstration that exogenous DNA sequences can be introduced into Drosophila germ lines has suggested new approaches that can be taken with other insect species.
Unfortunately, attempts at using Drosophila P-elements as vectors of exogenous DNA have not yet been successful in non-drosophilids. Alternative gene vectors will have to be discovered. Some possibilities for vectors are various insect viruses, such as baculoviruses, which might form stable transformations. Investigators are also searching in other insect species for transposable elements analogous to the Drosophila P-element.
Some of the techniques developed in molecular genetics that may be useful in autocidal control programs for insect pests include: nucleic acid hybridization, restriction mapping of chromosomes, nucleotide sequencing, cloning DNA sequences, insertion of recombinant DNA (transformation), and development of monoclonal antibodies.
Obstacles to progress in the use of molecular genetics for insect control are: 1) lack of a general vector for gene transfer, 2) lack of suitable selective criteria for useful loci, 3) uncertainty about what genetic modifications will prove most detrimental to a given species, 4) lack of knowledge of the basic genetic biology of most insect pests. None of these obstacles is insurmountable.
Integration into Pest Management Programs
The sterile insect release method and other autocidal control techniques are completely compatible with other types of insect control that might be used in IPM programs. In fact, E. F. Knipling has always insisted that autocidal control must be integrated with other measures in order to be used in the most effective way. The autocidal control technologies are most efficient and economical when pest populations are already at low levels. One reason for this is that these techniques generally involve release of laboratory (or factory) reared insects. Thus, the numbers of insects that can be reared will determine the size of the area of release. If less native insects are in the release zone, then a higher release ratio will be attained or a larger release area can be covered.
Any insect control measure that will reduce population densities either before or during the application of an autocidal control program will enhance the effectiveness of both procedures. For example, use of biocontrol organisms is generally most effective at high pest densities, but lose efficiency as pest densities decrease. Thus, a Sterile Insect Release Program would be a natural adjunct to any type of biological control program. This same reasoning applies to the use of insecticides to reduce high pest populations and the use of pheromones in confusion programs. Cultural control methods, which are generally used to reduce pest populations during their most vulnerable stages during the year, are completely compatible with the use of autocidal control programs because the latter programs would generally be used during the time of high reproductive potential of the target species. It is hard to think of an Integrated Pest Management Program that could not be enhanced by the addition of an autocidal control technique.
References
- Anonymous. 1993. Radiation Induced F1 Sterility in Lepidoptera for Area-wide Control. Proceedings of a Research Coordination Meeting, Phoenix, AZ, International Atomic Energy Agency, Vienna.
- Bartlett, A. C. 1990. Insect sterility, insect genetics, and insect control, pp. 279-287. In D. Pimentel [ed.], Handbook of Pest Management in Agriculture, Vol. II. CRC Press, Boca Raton, FL.
- Cockburn, A. F., A. J. Howells, and M. J. Whitten. 1989. Recombinant DNA technology and genetic control of pest insects, pp. 319 -349. In G. E. Russell [ed. Genetical and Biochemical Aspects of Invertebrate Crop Pests. Intercept Ltd., Andover, Hampshire, UK.
- Cox, H. C. 1978. Eradication of plant pests - pro's and con's. Bulletin of the Entomological Society of America. 24: 35 - 54.
- Davidson, G. 1974. Genetic Control of Insect Pests. Academic Press, New York, NY.
- Knipling, E. F. 1979. The Basic Principles of Insect Population Suppression and Management. U. S. Dept. of Agriculture. Agriculture Handbook No. 512. Washington, D. C.
- Knipling, E. F. 1985. Sterile insect technique as a screwworm control measure: The concept and its development, pp. 4-7. In O. H. Graham [ed.], Symposium on Eradication of the Screwworm from the United States and Mexico. Misc. Publ. Entomol. Soc. America 62, College Park, MD.
- Laster, M. L., D. D. Hardee, & J. C. Schneider. 1996. Heliothis virescens (Lepidoptera: Noctuidae) suppression by sterile backcross releases. J. Econ. Entomol. (in press).
- Pal, R. and Whitten, M. J. 1974. The Use of Genetics in Insect Control. American Elsevier, New York, NY.
- Staten, R. T., Rosander, R. W., and Keaveny, D. F. 1993. Genetic control of cotton insects, pp. 269-283. In Management of Insect Pests: Nuclear and Related Molecular and Genetic Techniques. Proc. Symp. Vienna, 1992), International Atomic Energy Agency, Vienna.
- Whitten, M. J. 1985. Conceptual basis for genetic control, pp. 465-528. In G. A. Kerkut and L. I. Gilbert [eds.], Comprehensive Insect Physiology, Biochemistry and Pharmacology. Pergamon Press, Oxford.