Roger H. Ratcliffe
USDA, ARS
Crop Protection and Pest Control Research Unit
West Lafayette, IN
The Hessian fly, Mayetiola destructor (Say) has a long history of pestilence in the United States. The insect was first found infesting wheat on Long Island, New York in 1779 and was believed to have been introduced by Hessian soldiers in straw bedding for horses during the American Revolutionary War. The Hessian fly now occurs in all major wheat-growing areas from the Atlantic Coast to the Great Plains. It also is found in the western United States in parts of California, Oregon, Washington and Idaho. The fly is capable of infesting and injuring all classes of wheat grown in the United States. Although wheat is the preferred host of the Hessian fly, barley, rye and triticale are other cultivated cereal crops occasionally infested. Several wild grasses may serve as hosts when these crops are not available.


When biotic and abiotic factors favor the insect's survival and development, populations may increase rapidly from one generation to the next. Although widespread outbreaks of the fly occur at irregular intervals in the U S, local outbreaks frequently can cause extensive crop losses. Consequently, when or where economic infestations may appear is not always predictable and methods for managing the Hessian fly are mostly preventive rather than remedial.


Management practices emphasize grower use of resistant wheat varieties, when available, and application of cultural control practices that include fall seeding of wheat after peak adult fly emergence (fly-free date) and destruction of volunteer wheat. Of these practices, resistant wheat varieties have provided the most reliable and economical form of management in much of the eastern U S soft winter wheat region and the Great Plains. Further information about cultural practices can be found in references cited at the end of this chapter.


Feeding injury by the Hessian fly is caused entirely by the larva. On young plants, the larva feeds beneath the leaf sheath at the base of the plant. The feeding mechanism of the larva and how it obtains food from the plant are not fully understood. A study of the larval mouthparts has revealed highly specialized mandibles that are probably used to inject salivary fluids into plants. These secretions are believed to contain enzymatic substances that inhibit plant growth and increase cell wall permeability that allows the larva to suck the juices from the plant.
No injury to plant tissue has been observed at feeding sites, although infested plants show a characteristic stunted appearance. The number of stems and leaves and foliar and root weight are reduced in wheat plants infested with Hessian fly larvae. Leaves of infested plants also appear more erect and are shorter and darker green than those of uninfested plants. When infestations are severe, stunting of seedlings occurs earlier and many of the young plants die after the larvae have matured.
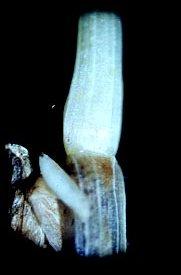

Larval feeding also reduces the winter hardiness of wheat plants that survive the fall infestation. This contributes to further loss during the winter. Heavily infested fields show areas of dead plants and thin stands. At lower infestation levels, seedlings may survive and develop new tillers that enable the plant to compensate for stunting of the main stem.
In the spring when plants are in the jointing stage, the larva feeds just above the node between the leaf sheath and culm. Feeding causes injury that prevents normal elongation of internodes and transport of nutrients to the developing spike. This injury reduces both quantity and quality of the grain. The most obvious damage occurs when infested culms break over at the weakened nodal areas. Much of the grain of broken culms is lost when the crop is harvested.
Hessian fly infestation in seedlings can be diagnosed by pulling up plants and examining the dead or stunted stems. Leaves should be peeled down to their points of attachment with the stem. The larva or flaxseed is located at the feeding site above the point of attachment. In older plants, the immature insect can be found within the shortened internode at the point of infestation, or, in lodged stems, just above the node where the stem broke.
Major programs to develop and release Hessian fly-resistant wheat varieties adapted to the Great Plains hard wheat and eastern U S soft winter wheat regions began in the 1920s and 1940s, respectively. The documented release of sixty Hessian fly-resistant varieties during the period from 1950 to 1983 is evidence of the success of these programs. In areas where resistant varieties have been grown for several years, losses by the Hessian fly have been reduced to <1%.
Numerous sources of resistance have been identified and incorporated into resistant wheat varieties, and varieties with new sources of resistance are continually developed and released when older varieties lose effectiveness. Resistance in these sources is dominant, partially dominant, or recessive, and conditioned by single, duplicate or multiple genetic factors derived from common wheat, Triticum aestivum L, durum wheat, T. turgidum L, the wild wheat, T. tauschii (Coss) Schmal., and rye, Secale cereale L. Twenty-eight major genes designated H1 through H28 have been described. Of the 28 genes, 8 were selected from common wheat, 13 from durum wheat, 5 from T. tauschii, and 2 from rye.
Antibiosis is the primary mechanism of resistance associated with these genes and is expressed as the death of the first-instar larvae within 2-3 days after establishment. The biochemical nature of antibiosis in wheat to the Hessian fly has not been determined for any gene. Research conducted to date has demonstrated no significant correlation between Hessian fly resistance and silica in sheaths of wheat plants or free amino-acids, organic acids, and sugars in plants.
Although the biochemical basis of resistance in wheat to Hessian fly is not known, research supports the hypothesis that hypersensitivity is the phenotypic basis of resistance in the Hessian fly-wheat interaction and involves "recognition" of an avirulence gene product or process by the plant in response to larval feeding.
Other genetic factors for resistance in wheat to the Hessian fly have been reported, but have been less utilized in breeding programs or variety development. These include resistance derived from the wheat varieties 'Kawvale' and 'Marquillo', which may include tolerance to larval feeding as well as antibiosis. Marquillo has been a durable source of resistance in several varieties for more than 30 years without biotype development. This type of resistance, along with antibiosis associated with the H3 gene, has been used extensively in the hard red winter wheat region of the Great Plains.
Tolerance also has been identified in some soft red winter wheat germplasm and is expressed as the ability of plants to avoid or recover from stunting following attack by Hessian fly larvae. Fly larvae survive and develop normally on 'tolerant' plants. Resistance in pubescent-leafed wheat varieties is due primarily to antixenosis, expressed as oviposition non-preference by Hessian fly females. Antibiosis of eggs or newly hatched larvae also may occur under hot, dry conditions due to increased desiccation of both life stages on leaves with greater pubescence.
The development of virulent biotypes of the Hessian fly poses the greatest threat to the permanence of resistance in wheat varieties. The growing of wheat varieties with high levels of resistance exerts a strong selection pressure on Hessian fly populations that favors races, or biotypes, capable of surviving and reproducing on resistant wheat. Therefore the biotype composition of Hessian fly populations within wheat growing regions changes with exposure to wheat varieties carrying specific resistance genes, and over time the effectiveness of varieties in preventing infestation by specific biotypes is reduced.
Sixteen Hessian fly biotypes, classified as 'GP' (Great Plains) and 'A' through 'O', have been identified on the basis of their differential response to the resistance genes H3, H5, H6, and H7H8 in wheat. The Great Plains biotype is considered to have been predominant in Hessian fly field populations prior to exposure to resistant wheat varieties and subsequent selection for virulence to resistance genes in these varieties. Hessian fly biotypes 'A' through 'O' differ in the number of resistance genes to which they express virulence. Biotype 'L' is the most virulent and can attack wheat varieties with any of these genes.
Major shifts in biotype composition and virulence to resistance genes in wheat occurred in Hessian fly populations throughout the eastern United States from the mid-1980's to late-1990's that greatly reduced effectiveness of all deployed genes. Information from field surveys conducted during this period demonstrated that Hessian fly biotype ‘L’ was predominant in populations throughout the Mid-west (Illinois, Indiana, Missouri, and Ohio), the mid-south (northern Alabama and Arkansas, and southern Tennessee), and the mid-Atlantic states of Delaware, Maryland, North Carolina, and Virginia. For this reason, wheat varieties carrying resistance genes H3, H5, H6, and H7H8 are ineffective in these areas. A soft winter wheat variety carrying Hessian fly resistance associated with the H13 gene was released jointly by Purdue University/USDA, ARS in 1998. The variety, INW9811, is resistant to Hessian fly populations from throughout the mid-west and mid-south soft winter wheat growing area.
Hessian fly populations in the lower southeastern US are predominantly biotype ‘O’, therefore resistance associated with the gene combination H7H8 is effective in southern Alabama, Georgia, and South Carolina, and northern Florida, in addition to resistance associated with H13. The composition of Hessian fly populations in eastern Washington and northern Idaho varies considerably from those found in the eastern US soft winter wheat region. Whereas biotype ‘GP’, which cannot survive on Hessian fly-resistant wheat, is absent in the eastern US, it makes up 25 to 75% of populations sampled in Washington and Idaho. Therefore, wheat varieties carrying all available sources of resistance remain moderately to highly effective in the Pacific Northwest.
The optimum use of present and future resistance genes in wheat breeding programs is dependent on the development of gene deployment strategies that improve the durability of resistance in wheat varieties exposed to Hessian fly populations. Breeding strategies incorporating the use of moderately resistant genes or combinations of genes with different levels of resistance, genes that respond differently to abiotic factors such as temperature, and selective use of genes depending upon the biotype composition of Hessian fly populations are being explored. Molecular markers associated with resistance genes in wheat continue to be identified and will be of value in developing marker assisted selection to complement conventional selection procedures in breeding programs. Marker assisted selection will be very useful in stacking genes for Hessian fly resistance in wheat varieties. Implementation of these strategies will reduce selection pressure on fly populations that would favor the increase of virulent genotypes.
Selected References
- Buntin, D. G. and J. W. Chapin. 1990. Biology of Hessian fly (Diptera: Cecidomyiidae) in the southeastern United States: Geographic variation and temperature-dependent phenology. J. Econ. Entomol. 83:1015-1024.
- Cambron, S. E., F. L. Patterson, H. W. Ohm, R. H. Ratcliffe, and G. G. Safranski. 1995. Genetic analyses of Hessian fly resistance in eight durum wheat introductions. Crop Sci. 35:708-714.
- Dweikat, I., H. Ohm, S. Mackenzie, F. Patterson, S. Cambron, and R. Ratcliffe. 1994. Association of a DNA marker with Hessian fly resistance gene H9 in wheat. Theor. Appl. Genet. 89:964--968.
- Formusoh, E. S., J. H. Hatchett, W. C. Black IV, and J. J. Stuart. 1996. Sex-linked inheritance of virulence against wheat resistance gene H9 in the Hessian fly. Ann. Entomol. Soc. Amer. 89:428-434.
- Friebe, B., J. H. Hatchett, R. G. Sears, and B. S. Gill. 1990. Transfer of Hessian fly resistance from 'Chaupon' rye to hexaploid wheat via a 2BS/2RL wheat-rye chromosome translocation. Theor. Appl. Genet. 79:385-389.
- Gagne, R. J. and J. H. Hatchett. 1989. Instars of the Hessian fly (Diptera: Cecidomyiidae). Ann. Entomol. Soc. Am. 82:73-79.
- Gallun, R. L. and L. P. Reitz. 1971. Wheat cultivars resistant to Hessian fly. USDA, ARS, Production Res. Rept. 134:16.
- Grover, P. B., Jr. 1995. Hypersensitive response of wheat to the Hessian fly. Entomol. Exp. Appl. 74:283-294.
- Hatchett, J. H., G. L. Kreitner, and R. J. Elzinga. 1990. Larval mouthparts and feeding mechanism of the Hessian fly (Diptera: Cecidomyiidae). Ann. Entomol. Soc. Am. 83:1137-1147.
- Hatchett, J. H., T. J. Martin, and R. W. Livers. 1981. Expression and inheritance of resistance to Hessian fly in synthetic hexaploid wheats derived from Triticum tauschii (Coss) Schmal. Crop Sci. 21:731-734.
- Hatchett, J. H., R. G. Sears, and T. S. Cox. 1993. Inheritance of resistance to Hessian fly in rye and wheat-rye translocation lines. Crop Sci. 33:730-734.
- McKay, P. A. and J. H. Hatchett. 1984. Mating behavior and evidence of a female sex pheromone in the Hessian fly, Mayetiola destructor (Say) (Diptera: Cecidomyiidae). Ann. Entomol. Soc. Amer. 77:616-620.
- Ohm, H. W., H. C. Sharma, F. L. Patterson, R. H. Ratcliffe, and M. Obanni. 1995. Linkage relationships among genes on wheat chromosome 5A that condition resistance to Hessian fly. Crop Sci. 35:1603-1607.
- Ratcliffe, R .H., H. W. Ohm, F. L. Patterson, S. E. Cambron, and G. G. Safranski. 1996. Response of resistance genes H9 to H19 in wheat to Hessian fly (Diptera: Cecidomyiidae) laboratory biotypes and field populations from the Eastern United States. J. Econ. Entomol. 89:1309-1317.
- Ratcliffe, R. H. and J. H. Hatchett. 1997. Biology and genetics of the Hessian fly and resistance in wheat. p. 47-56. In K. Bondari [ed], New Developments in Entomology. Publisher, Research Signpost, Scientific Information Guild, Trivandrum, India.
- Ratcliffe, R. H., S. E. Cambron, K. L. Flanders, N. A. Bosque-Perez, S. L. Clement, and H. W. Ohm. 2000. Biotype composition of Hessian fly (Diptera: Cecidomyiidae) populations from the southeastern, Midwestern, and northwestern United States and virulence to resistance genes in wheat. J. Econ. Entomol. 93:1319-1328.