G. Michael Chippendale
Chippendale Consulting, LLC
29 S. 9th Street, Suite 209, Columbia, Missouri 65201
Clyde E. Sorenson
Department of Entomology, Box 7630
North Carolina State University, Raleigh, NC 27695
Abstract
The distribution, life stages, phenology, economic importance, and management of the southwestern corn borer, Diatraea grandiosella, are described. Over this century, the southwestern corn borer has expanded its range from Mexico into the maize-producing regions of the southern United States where it has become a serious pest. The modern practices of conservation tillage, which leaves undisturbed the overwintering habitat, and extensive irrigation, which increases maize acreage within the insect's range, tend to favor the buildup of populations. At present, cultural methods combined with precisely timed insecticide applications offer the most practical ways to decrease populations and to limit losses to the southwestern corn borer. Early planting of maize minimizes dead-heart injury because plants tend to reach the tassel stage of development by the time first generation larvae hatch, and fall or spring plowing or discing destroys diapause larvae. Improved management are likely to come from the development of varieties of maize, through classical breeding and biotechnology, which are resistant to feeding by first and second generation larvae.
Introduction
The southwestern corn borer, Diatraea grandiosella Dyar (Lepidoptera; Pyralidae), is able to grow and develop successfully on only a relatively few species of wild or cultivated grasses, with maize, Zea mays, being the preferred host. The insect is found in Mexico and the United States. In Mexico, the species has been reported to be present in seven southcentral states, in four northwestern states, and in the Rio Grande Valley, Apodaca, and Rio Brava of the northeast. The species does not appear to be present east of the northern state of Chihuahua or in the central plateau of Mexico (Abarca et al., 1958; Elias, 1970). The first official records of the presence of D. grandiosella in the United States are in 1913 from Lakewood and Las Palomas, New Mexico (Davis et al., 1933). While the insect may have entered the United States much earlier, records are unavailable because the species was not named until 1911 (Dyar, 1911). Eastwardly migration rates of 13 miles per year (1913 to 1931), 20 miles per year (1932 to 1953), and 35 miles per year (1954 to 1964) have been estimated (Fairchild et al., 1965). The insect is now established in 13 southern states (Davis et al., 1933; Chippendale and Reddy, 1974; Chippendale and Cassatt, 1985). The current northern limits of its distribution are southcentral Kansas (380 N latitude), and the species has reached to the western edge of Georgia (850 W longitude). The primary factors controlling population levels of D. grandiosella at its northern limits appear to be sub-freezing temperatures and natural enemies (Popham et al., 1991).
The genetic differentiation accompanying the range expansion of D. grandiosella from Mexico into the United States has been investigated (McCauley et al., 1995). A moderately large genetic differentiation was observed among populations from Missouri, Mississippi, and Texas and a population from Arizona. All four of these populations differed significantly from a population from southern Mexico. A cluster analysis of the co-ancestral coefficients calculated between pairs of populations showed a hierarchy of genetic differentiation which agrees with the known geographical dispersal of the species from Mexico. This finding is consistent with the reported phenotypic variation of D. grandiosella from Mexico and the United States (Kikukawa et al., 1984).
Several reviews and annotated bibliographies are available about the southwestern corn borer (Davis et al., 1933; Walton and Bieberdorf, 1948; Wilbur et al., 1950; Rolston, 1955; Morrison et al., 1977; Chippendale, 1979; and Chippendale and Cassatt, 1985).
Economic Losses to Maize
The southwestern corn borer causes losses to maize in the southwestern and southern plains states of the US which have been estimated at several million dollars annually (Morrison et al., 1977). In addition, the destructiveness of the insect has caused a change in agronomic practices in portions of several states which have high populations of D. grandiosella. These changes include early planting and fall discing or plowing, and in extreme cases a reduction in maize acreage as farmers have turned to other crops, usually sorghum in Kansas and Oklahoma. For example, maize acreages were reduced by as much as 50% per year when the insect first became a destructive pest in south-central Kansas in the early 1940s (Wilbur et al., 1950). While the insect generally causes less economic loss in sorghum than it does in maize, periodically it is a troublesome pest of sorghum in Arizona. Infested sorghum plants show retarded growth and reduced grain production, and yield losses up to 50% have been reported (Gerhardt et al., 1972).
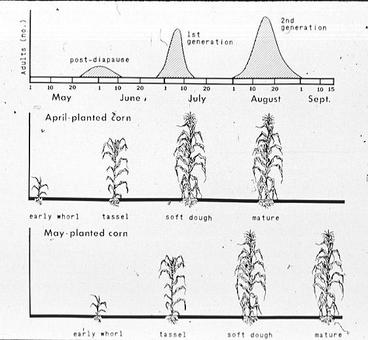
Economic losses occur in silage maize when the feeding damage of southwestern corn borers reduces plant vigor to the extent that vegetative growth is significantly retarded, and losses in sweet and field maize occur when ear production is retarded. The lodging of mature maize plants due to the girdling activity of larvae preparing to diapause in the stalk crown may cause the loss of entire plants to a mechanical harvester (Fig. 1). Under Missouri conditions, harvesting maize before mid-September usually prevents most yield losses from the lodging of girdled plants. Typically, most yield losses occur in irrigated maize which is planted and harvested late. Under these conditions plants are susceptible to dead-heart injury by first generation larvae, and to a high rate of lodging following the girdling activity of fully grown larvae entering diapause (Fig.2).

Scott and Davis (1974) observed that the feeding of first generation larvae reduced plant height by about 16 cm. They also recorded grain yield losses of up to 29% in plants infested with first and second generation larvae. Although these losses resulted from a decrease in the number of kernels per plant, ears harvested from girdled and lodged maize have also been shown to have a lower dry weight and higher water content than those from uninfested maize (Chada et al., 1965; Daniels, 1977).
Life Stages

Figure 3 illustrates the life stages of D. grandiosella. Eggs are laid on both the upper and lower surfaces of the leaves and stalk of the host plant. Female moths typically prefer maize plants of intermediate size as sites for oviposition. Each female lays from 100 to 400 eggs, either singly or overlapping one another in masses of several eggs. Immediately after being oviposited, each egg is flattened, elliptical, about 1 mm wide, and a uniform pale yellow. Within 36 hrs each egg develops three transverse orange bands which help to distinguish fertile eggs of D. grandiosella from those of several other maize feeding insects, including the European corn borer, Ostrinia nubilalis.

Southwestern corn borers show a seasonal polymorphism. Summer-form larvae (non-diapause) are off-white with black pinacula, whereas winter-form larvae (diapause) are immaculate, i.e. are uniformly light yellow due to their pigment-free pinacula. After passing through at least five stages, non-diapause larvae pupate without any prior loss of cuticular pigments. In contrast, fully grown prediapause larvae molt from the spotted to the immaculate morph at the onset of diapause. Larvae show a distinct behavioral pattern as they prepare for diapause by migrating to the base of the stalk below ground level where they prepare an overwintering cell (Fig 4).
In the process of preparing this cell and an exit hole for the escape of the moth, they may girdle the plant a few cm above ground level. Because of this behavior and their tendency towards cannibalism, usually only one larva overwinters per plant, even though each maize plant can support the growth of several larvae.
In preparation for pupation, southwestern corn borers spin small amounts of silk, but a protective cocoon is not formed around the pupa. Male pupae are usually smaller than female pupae. The pupa is the earliest metamorphic stage in which sex can be determined externally; the genital opening of the male is located nearer to the anal opening than is that of the female.
Phenology
The life cycle of the southwestern corn borer is tightly synchronized with that of its host plants. This synchronization is achieved by the intervention of the larval diapause which permits the insect to survive in a dormant state from September to May, when host plants are not available. The insect uses day length and temperature to program both the induction and termination of its diapause. The relationship between day length, temperature, and the number of generations per year of D. grandiosella in southeast Missouri is shown in a photothermogram (Fig. 5) (Takeda and Chippendale, 1982).
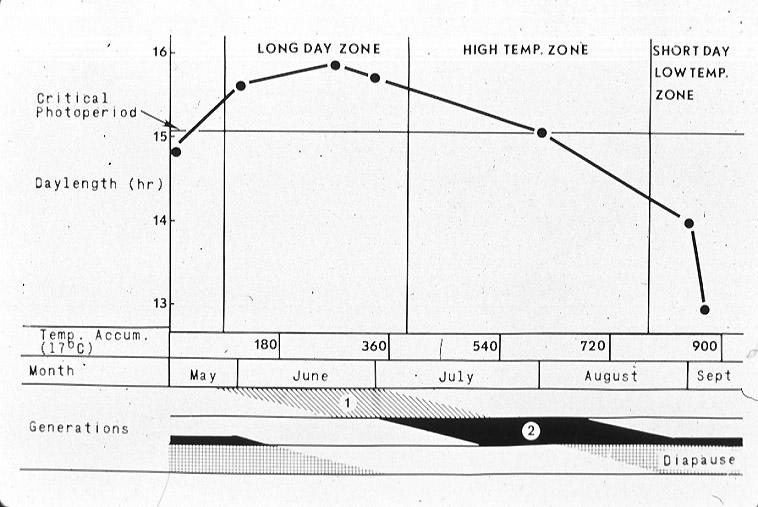
The conditions present at this latitude allow two complete generations per year and a partial third generation. Second and third generation larvae exposed to short days and low temperatures beginning in mid-August enter diapause.
An important factor regulating the phenology of D. grandiosella is the relationship between its life stages and the availability of its host plant for oviposition and food (Fig. 2). Maize plants take about 18 weeks to mature, of which nine weeks are required for both vegetative growth and grain development (Hanway, 1971). In contrast, one complete generation of the insect requires from six to eight weeks, depending upon the prevailing local conditions. The following ranges of generation times have been estimated: 42 to 49 days in Sinaloa, Mexico (Abarca et al., 1958) 40 to 50 days in Arizona (Davis et al., 1933), 38 to 56 days in Oklahoma (Walton and Bieberdorf, 1948), 45 days in Kansas (Wilbur et al., 1950), and 41 days in Arkansas (Rolston, 1955). Since maize is the most acceptable site for oviposition and for larval feeding during its first nine weeks, three complete generations per year are found only when the generation times of the host plant and the corn borer are highly synchronized.
Integrated Pest Management
While the southwestern corn borer is very difficult to manage, several tactics can be brought to bear on economic infestations. Larvae of the first generation occasionally cause significant damage to whorl-stage maize, but those of the second generation, which attack during the early reproductive phases of the maize crop, typically cause more damage because they are present in much higher numbers (Fig. 2). Specific problems associated with the second generation include a sometimes protracted egg deposition period, the brief time larvae remain outside of the plant, and the plant architecture during this growth phase. However, several tactics, used in concert, can help decrease the impact of southwestern corn borer infestations on maize yields. Perhaps the most important current management strategies are cultural measures and biological control. Judicious use of insecticides based on field sampling (scouting) may also provide some decrease in damage. Advances in both traditional breeding for host plant resistance and in genetically engineered resistance hold great promise for improving the ability to manage populations of the insect.
The sex pheromone of D. grandiosella has been identified as a mixture of (Z)9-hexadecenal, (Z)11-hexadecenal, and (Z)13-octadecenal (Hedin et al., 1986), and effective commercial lures are available (Knutson et al., 1988). Monitoring adult populations can provide important information about the timing of infestations and scouting activities. Pheromone traps have been found effective for detecting the onset of moth flights (Knutson et al., 1987). Plastic bucket traps appear to be the most efficient and economical type (Goodenough et al., 1989). Light traps are attractive to moths (Schenk and Poston, 1979), but pheromone traps probably offer increased flexibility in deployment and are much less expensive.
Scouting for southwestern corn borers should focus on eggs, because larvae are not easily detected and remain on the exterior of the plant for only a few days (Davis et al., 1972). Scouting recommendations in Missouri call for examining five, 20 plant samples for the presence of eggs and small larvae. Sequential and binomial sampling plans for second generation eggs and small larvae have been developed (Overholt et al., 1990). A nominal economic threshold of 25% plants with eggs or small larvae has been adopted by several states where D. grandiosella is a pest. Refinements of thresholds based on an improved understanding of infestation damage relationships are needed. In Kansas, a phenology model (Whitworth and Poston, 1979) is used to assist with the timing of spray applications.
Cultural Control
Cultural methods are a practical and economical way to suppress larval populations of D. grandiosella. These methods have to be adapted to local conditions, and work best when practiced over a reasonably large area because the moths readily migrate from infested fields. Early planting and fall or spring plowing or discing tend to decrease populations (Wilbur et al., 1950; Rolston, 1955). Early planting minimizes the damage (dead heart) caused by first generation larvae to the foliage and meristematic tissues of maize because the plants have already passed their critical growth stage before significant numbers of larvae begin to feed. In addition, early planted maize can be harvested in August before many fully grown pre-diapause larvae have girdled the mature plants and caused yield losses through lodging of the plants. For example, maize planted in April or early May in Missouri usually escapes dead-heart injury because it has passed the critical growth stage by the time large numbers of first generation larvae begin to feed ( Fig. 2). Maize planted on April 9 and artificially infested on May 15 suffered 36% dead-heart injury as well as disastrous yield losses. By contrast, maize planted on the same date, but artificially infested on May 26, escaped dead-heart injury and extreme grain yield losses (Arbuthnot et al., 1958).
A useful cultural practice is fall or early spring plowing or discing to bury or uproot maize stubble and to destroy the overwintering habitat of the southwestern corn borer (Fig. 4) (Daniels and Chedester, 1975). This practice increases mortality by exposing larvae to natural hazards, and therefore decreases the number of spring moths. The practice can, however, be counterproductive in areas where soil erosion is a problem. The practices of no-tillage or minimum-tillage and of planting winter wheat over maize stubble tend to protect the diapause larvae in their overwintering habitat. Other sound agronomic practices include growing vigorous locally adapted hybrids in fertile soil, and harvesting the grain early to reduce yield losses due to stalk lodging caused by the girdling behavior of pre-diapause larvae.
Chemical Control
Foliar applications of insecticides require precise timing to be effective against the southwestern corn borer because the insect spends much of its larval life within the stalk. Early planted maize usually escapes significant damage, and while late planted maize might be heavily infested, insecticide treatments must be timed carefully to reach the larvae before they enter the stalk. Judicious early planting and harvesting of maize can, therefore, obviate a need for insecticide treatments. Since most larvae enter the stalk within 12 to 14 days after hatching, insecticides must usually be applied within a week of detecting larvae of the first generation. Screening tests have shown that only a few liquid or granular foliar insecticides are effective against the insect.
The current chemical control recommendations in Missouri for the southwestern corn borer are directed at second generation oviposition, or when scouting shows that 25% of the plants are infested with eggs or larvae. Eggs and larvae of the second generation are targeted because of the potential for economic losses, but the larvae of this generation are difficult to control with a single application of insecticide because moths are present from early July to mid August. Broadcast treatments of the pyrethroids esfenvalerate and permethrin, and the carbamates, carbofuran and carbaryl, are recommended.
Host Plant Resistance
For many years entomologists and plant breeders have been working on developing varieties of maize that show resistance to the southwestern corn borer. This is a complex research topic because lines resistant to feeding damage from first generation larvae are not necessarily resistant to that from subsequent generations (Barry and Darrah, 1978). First generation larvae feed on whorl stage plants, whereas second generation larvae feed on ears, ear shoots and husks of plants that have already tasseled.
An early release was the inbred line MP496 which showed some resistance to damage by first generation larvae (Davis et al., 1973). More recently, hybrids based on MP496 and other resistant germ plasm have shown significant resistance to larval feeding (Davis and Williams, 1986). The resistance of these lines appears to be based on antibiosis, but these lines also display a non-preference for oviposition and larval feeding (Davis et al., 1989, Ng et al., 1990).
The recent development of maize lines genetically engineered to produce the endotoxin of Bacillus thuringiensis (Bt) holds promise as a tool for management of the southwestern corn borer. A field trial conducted in southeast Missouri in 1996 showed virtually no tunneling damage from European or southwestern corn borers in two Bt transgenic maize cultivars tested. The susceptible control had the lowest yield, but the yield was not significantly different from one of the cultivars tested (D. Barry, unpubl. data).
Biological Control
The highest rate of natural mortality typically occurs among overwintering southwestern corn borers in the stalk crown below ground level. Field observations have shown overwintering mortality ranging from about 50% to>95%. These high mortality rates result in relatively few spring moths and first generation larvae. However, as Wilbur et al. (1950) have noted, a winter survival rate of 2% in heavily infested areas is adequate to restore population levels in the second generation.
In Missouri, the southwestern corn borer is at the northern limits of its distribution and the primary factors controlling populations appear to be freezing temperatures and bird predators. The detrimental effects of low midwinter temperatures are enhanced in wet clay soils by inoculative freezing. The higher mortality of diapause larvae in wet clay or loam soils than in porous sandy soils is well documented (Wilbur et al., 1950; Langille, 1975).
The migration of D. grandiosella across the southern maize belt has been aided by the absence or low populations of natural enemies necessary to keep field populations in check. The hymenopteran, Trichogramma minutum, is an effective late-season parasite. The wasps are most abundant in late summer and are most effective in parasitizing eggs of the third generation, thereby decreasing the number of diapause larvae. High rates of parasitism of third generation eggs of D. grandiosella have been observed in Arkansas, Arizona, and Missouri (A. J. Keaster, unpubl. inform.). In a study conducted on the High Plains of Texas, at least two exotic parasitoids showed promise as biological control agents for D. grandiosella (Overholt and Smith, 1990).
Pathogens cause considerable mortality of diapause southwestern corn borers. Both fungal and bacterial pathogens have been observed to invade diapause larvae in their overwintering cells (Davis et al., 1933; Langille, 1975). A fungus of Beauveria sp. kills up to 6% of diapause larvae in Missouri by entering the hemocoel of diapause larvae in the fall. Initially, attacked larvae turn pink, and then after a few days turn white after the fungal hyphae have spread over the cuticle. Similarly, a bacterium of Bacillus sp. decreases fall populations of diapause larvae by about 10%(Langille, 1975). Although larvae have been shown to be susceptible to Bt under laboratory conditions (Sikorowski and Davis, 1970), preparations of Bt are not being used commercially against the southwestern corn borer. However, transgenic Bt maize has considerable potential use against this insect ( B. D. Barry, pers.comm.).
Southwestern corn borers that overwinter in stalks which disintegrate as a result of fungal induced stalk rot are more likely to be attacked by pathogens than are larvae in intact stalks. The decaying maize plant provides a suitable environment for the growth of microorganisms which may ultimately invade the diapause larvae. However, any conclusions about the lethal effects of assumed pathogens must be drawn from controlled laboratory studies.
Bird predation in maize stubble is important in suppressing populations of diapause southwestern corn borers in Arkansas (Wall and Whitcomb, 1964), Louisiana (Floyd et al., 1969), Mississippi (Davis et al., 1973), and Missouri (Langille, 1975). The yellow-shafted flicker, Colaptes auratus is the most important predator. The flicker seeks larvae in stalk crowns of plants which show external signs (entrance holes, girdled stalk) of their presence. The bird pecks a hole about 0.5 cm in diameter near ground level among the brace roots, and in so doing gains entrance to the overwintering cell. Most predation occurs from December to early March. By late March the population density of the insect has declined and other food sources for the flicker are becoming available.
Large southwestern corn borer larvae are cannibalistic. This trait is a significant factor in the population dynamics of the second generation (Knutson and Gilstrap, 1990). The propensity for cannibalism may vary among populations (Tarpley et al., 1993).
Acknowledgement
We thank our colleagues who have contributed to the results summarized in this report: Dean Barry, Felix Breden, Katherine Connor, Jack Dillwith, Paula Ezell, Milon George, Jeff House, Shigeru Kikukawa, David McCauley, Holly Popham, A. S. Reddy, Nathan Schiff, Makio Takeda, and Megan Tarpley. This article is a contribution from the Missouri Agricultural Experiment Station, paper no. 12,587.
References
- Abarca, M., A. Iturbe, and F. Caceres. (1958) The sugarcane borers in Mexico. An attempt to control them through parasites. Proc. 10th Int. Cong. Ent. pp. 827-834.
- Arbuthnot, K. D., R. R. Walton, and J. S. Brooks. (1958) Reduction in corn yield by first generation southwestern corn borers. J. Econ. Ent. 51, 747-749.
- Archer, T. L., E. D. Bynum, Jr., and A. Knutson. (1983) Winter management of the southwestern corn borer (Lepidoptera: Pyralidae), using several cultural practices on different dates. J. Econ. Ent. 76: 872876.
- Barry, D., and L. L. Darrah. (1978) Identification of corn germplasm resistant to the first generation of southwestern corn borer. J. Econ. Ent. 71, 877-879.
- Chada, H. L., D. L. Bailey, and R. R. Walton. (1965) Corn yield losses from southwestern corn borer infestation as related to harvesting methods. Okla. Agr. Exp. Sta. Process. Ser. P-496. 8pp.
- Chippendale, G. M. (1979) The southwestern corn borer: case history of an invading insect. Research Bulletin UMC Agric. Exp. Sta. no. 1031. 52pp.
- Chippendale, G. M. (1982) Insect diapause, the seasonal synchronization of life cycles, and management strategies. Ent. Exp. Appl. 31:24-35.
- Chippendale, G. M. and A. S. Reddy. (1974) Diapause of the southwestern corn borer, Diatraea grandiosella: low temperature mortality and geographical distribution. Environ. Ent. 3, 233-238.
- Chippendale, G. M. and K. L. Cassatt. (1985) Case history of the southwestern corn borer. II. Annotated bibliography, 1977 to 1985. Misc. Publ. Ent. Soc. Amer. no. 60, pp. 1-30.
- Daniels, N. E. (1977) Southwestern corn borer. pp. 26-32 in Corn Prod. Symp. Texas A&M Univ. Agr. Res. Ext. Cent. Amarillo. 38 pp.
- Daniels, N. E. and L. D. Chedester. (1975) Cultural control and biological studies of the southwestern corn borer. Texas Agr. Exp. Stat. Prog. Rep. 3357C. 8pp.
- Davis, E.G., J. R. Horton, C. H. Gable, E. V. Walter, R. A. Blanchard, and C. Heinrich. (1933) The southwestern corn borer. USDA Tech. Bull. 388. 61 pp.
- Davis, F. M., C. A. Henderson, and G. E. Scott. (1972) Movements and feeding of larvae of the southwestern corn borer on two stages of corn growth. J. Econ. Ent. 65: 519521.
- Davis, F. M., C. A. Henderson, and T. G. Oswalt. (1973) Mortality of overwintering southwestern corn borers in Mississippi. Environ. Ent. 2, 86-88.
- Davis, F. M., and W. P. Williams. (1986) Survival, growth and development of southwestern corn borer (Lepidoptera: Pyralidae) on resistant and susceptible maize hybrids. J. Econ. Ent. 79:847851.
- Davis, F. M., S. S. Ng, and W. P. Williams. (1989) Mechanisms of resistance in corn to leaf feeding by southwestern and European corn borer (Lepidoptera: Pyralidae). J. Econ. Ent. 82:919922.
- Dyar, H. G. (1911) The American species of Diatraea Guilding. Ent. News 22, 199-207.
- Elias, L. A. (1970) Maize resistance to stalk borers in Zeadiatraea Box and Diatraea Guilding at five localities in Mexico. Ph.D. Dissertation. Kans. State Univ. 172 pp. Univ. Microfilms no. 70-16,627
- Fairchild, M. L., A. J. Keaster, and C. C. Burkhardt. (1965) The southwestern corn borer. Proc. Hybrid Corn Industry-Research Conf. 20, 111-117.
- Floyd, E. H., L. Mason, and S. Philips. (1969) Survival of overwintering southwestern corn borers on corn stalks in Louisiana. J. Econ. Ent. 62, 1916-1919.
- Gerhardt, P. D., L. Moore, J. F. Armstrong, and L. J. Kaspersen. (1972) Southwestern corn borer control in grain sorghum. J. Econ. Ent. 65, 491-494.
- Goodenough, J. L., A. E. Knutson, and F. M. Davis. (1989) Trap comparisons and behavioral observations for the male southwestern corn borer (Lepidoptera: Pyralidae). J. Econ.Ent. 82: 14601465.
- Hanway, J. J. (1971) How a corn plant develops. Iowa State Univ. Special Report no. 48. 17 pp.
- Hedin, P. A., F. M. Davis, J. C. Dickens, M. L. Burks, T. G. Bird, and A. E. Knutson. (1986) Identification of the sex attractant pheromone of the southwestern corn borer Diatraea grandiosella Dyar. J. Chem. Ecol. 12: 20512063.
- Kikukawa, S., J. W. Dillwith, and G. M. Chippendale. (1984). Characteristics of larvae of the southwestern corn borer, Diatraea grandiosella, obtained from populations present in tropical and temperate regions. J. Insect Physiol. 30, 787-796.
- Knutson, A. E., F. M. Davis, T. G. Bird, and W. P. Morrison. (1987) Monitoring southwestern corn borer, Diatraea grandiosella Dyar with pheromone traps. Southwest Ent. 12: 6571.
- Knutson, A. E., F. M. Davis, P. A. Hedin, and V. A. Phillips. (1988) Field evaluation of eight substrates for dispensing pheromone of the southwestern corn borer (Lepidoptera: Pyralidae). J. Econ. Ent. 81: 14741477.
- Knutson, A. E., and F. E. Gilstrap. (1989) Direct evaluation of natural enemies of the southwestern corn borer (Lepidoptera: Pyralidae) in Texas corn. Environ. Ent.18: 732-739.
- Knutson, A. E., and F. E. Gilstrap. (1990) Life tables and population dynamics of the southwestern corn borer (Lepidoptera: Pyralidae) in Texas corn. Environ. Ent.19: 684-696.
- Langille, R. H. (1975) Observations on the overwintering survival and spring development of the southwestern corn borer, Diatraea grandiosella Dyar. Ph.D. Dissertation. Univ. Missouri. 153 pp. Univ. Microfilms no. 76-1027.
- McCauley, D. E., N. Schiff, F. J. Breden, and G. M. Chippendale. (1995). Genetic differentiation accompanying range expansion of the southwestern corn borer (Lepidoptera: Pyralidae). Ann. Ent. Soc. Amer. 88,357-361.
- Morrison, W. P., D. E. Mock, J. D. Stone, and J. Whitworth. (1977) A bibliography of the southwestern corn borer, Diatraea grandiosella Dyar. Bull. Ent. Soc. Amer. 23, 185-190.
- Ng, S. S., F. M. Davis, and W. P. Williams.(1990) Ovipositional response of southwestern corn borer (Lepidoptera: Pyralidae) and fall armyworm (Lepidoptera: Noctuidae) to selected maize hybrids. J. Econ. Ent. 83: 15751577.
- Overholt, W. A., and J.W. Smith, Jr.(1990) Colonization of six exotic parasites (Hymenoptera) against Diatraea grandiosella (Lepidoptera: Pyralidae) in corn. Environ. Ent. 19: 18891902.
- Overholt, W. A., A. E. Knutson, J. W. Smith, Jr., and F. E. Gilstrap.(1990) Distribution and sampling of southwestern corn borer (Lepidoptera: Pyralidae) in preharvest corn. J. Econ. Ent. 83: 13701375.
- Popham, H. J. R., M. F. George and G. M. Chippendale. (1991) Cold hardiness of larvae of the southwestern corn borer, Diatraea grandiosella. Ent. Exp. Appl. 58:251-260.
- Rolston, L. H. (1955) The southwestern corn borer in Arkansas. Arkansas Agr. Exp. Sta. Bull. 533. 40pp.
- Schenk, J. L., Jr., and F. L. Poston. (1979) Adjusting lighttrap samples of the southwestern corn borer for ageclass bias. Ann. Ent. Soc. Amer. 72: 746748.
- Scott, G. E. and F. M. Davis. (1974) Effect of southwestern corn borer feeding on maize. Agron. J. 66, 773-774.
- Scott, G. E. and F. M. Davis. (1978) Second-brood damage by southwestern corn borer in a corn diallel cross. Crop Sci. 18, 355-336.
- Sikorowski, P. and F. M. Davis. (1970) Susceptibility of larvae of the southwestern corn borer, Diatraea grandiosella, to Bacillus thuringiensis. J. Invert. Pathol. 15, 131-132.
- Takeda, M. and G. M. Chippendale. (1982) Phenological adaptations of a colonizing insect: the southwestern corn borer, Diatraea grandiosella. Oecologia. 53:386-393.
- Tarpley, M. D., F. Breden, and G. M. Chippendale. (1993) Genetic control of geographic variation for cannibalism in the southwestern corn borer, Diatraea grandiosella. Ent. Exp. Appl. 66: 145-152.
- Wall, H. C. and W. H. Whitcomb. (1964) The effect of bird predators on winter survival of the southwestern and European corn borers in Arkansas. J. Kans. Ent. Soc. 37, 187-192.
- Walton R. R. and G. A. Bierberdorf. (1948) Seasonal history of the southwestern corn borer, Diatraea grandiosella Dyar, in Oklahoma; and experiments on methods of control. Okla. Agr. Exp. Sta. Tech. Bull. T-32. 23pp.
- Whitworth, R. J., and F. L. Poston. (1979) A thermal-unit accumulation system for the southwestern corn borer. Ann. Ent. Soc. Amer. 72: 253-255.
- Wilbur, D. A., H. R. Bryson, and R. H. Painter. (1950) Southwestern corn borer in Kansas. Kans. Agr. Exp. Sta. Bull. 339 46pp.